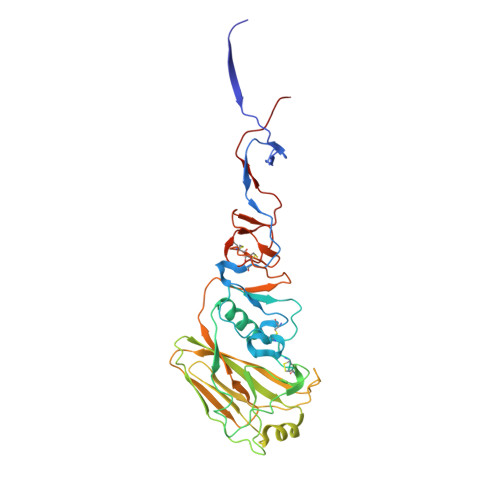
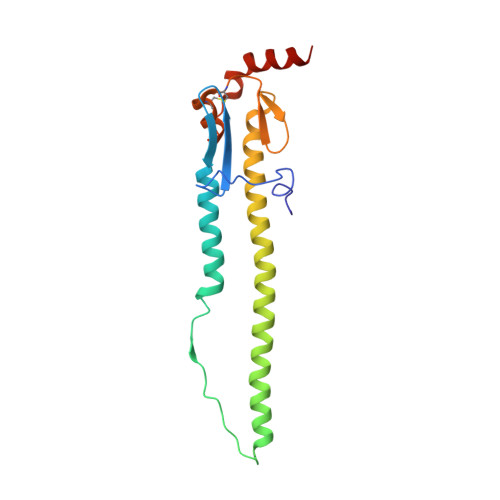
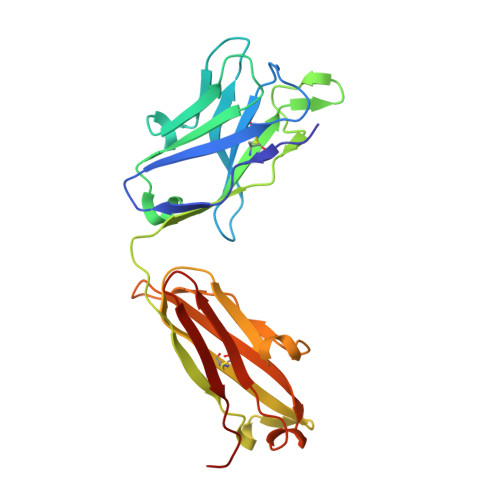
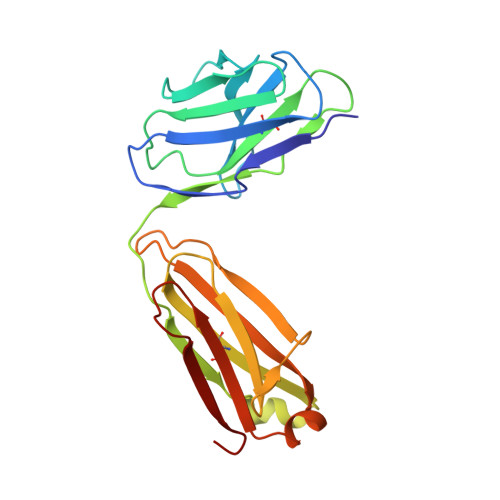
Maturation of germinal center B cells after influenza virus vaccination in humans.
McIntire, K.M., Meng, H., Lin, T.H., Kim, W., Moore, N.E., Han, J., McMahon, M., Wang, M., Malladi, S.K., Mohammed, B.M., Zhou, J.Q., Schmitz, A.J., Hoehn, K.B., Carreno, J.M., Yellin, T., Suessen, T., Middleton, W.D., Teefey, S.A., Presti, R.M., Krammer, F., Turner, J.S., Ward, A.B., Wilson, I.A., Kleinstein, S.H., Ellebedy, A.H.(2024) J Exp Med 221
- PubMed: 38935072
- DOI: https://doi.org/10.1084/jem.20240668
- Primary Citation of Related Structures:
8TXM, 8TXP, 8TXT, 8TY7 - PubMed Abstract:
Germinal centers (GC) are microanatomical lymphoid structures where affinity-matured memory B cells and long-lived bone marrow plasma cells are primarily generated. It is unclear how the maturation of B cells within the GC impacts the breadth and durability of B cell responses to influenza vaccination in humans. We used fine needle aspiration of draining lymph nodes to longitudinally track antigen-specific GC B cell responses to seasonal influenza vaccination. Antigen-specific GC B cells persisted for at least 13 wk after vaccination in two out of seven individuals. Monoclonal antibodies (mAbs) derived from persisting GC B cell clones exhibit enhanced binding affinity and breadth to influenza hemagglutinin (HA) antigens compared with related GC clonotypes isolated earlier in the response. Structural studies of early and late GC-derived mAbs from one clonal lineage in complex with H1 and H5 HAs revealed an altered binding footprint. Our study shows that inducing sustained GC reactions after influenza vaccination in humans supports the maturation of responding B cells.
Organizational Affiliation:
Department of Pathology and Immunology, Washington University School of Medicine, St Louis, MO, USA.