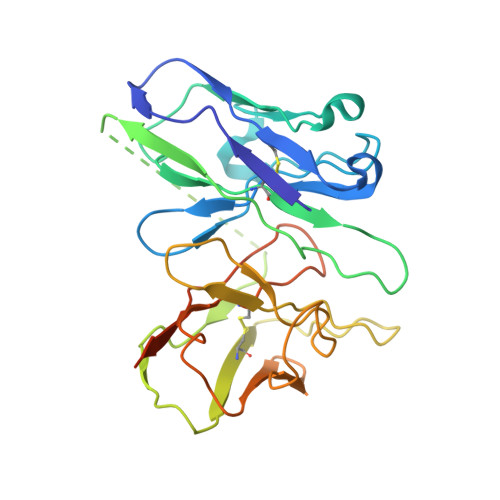
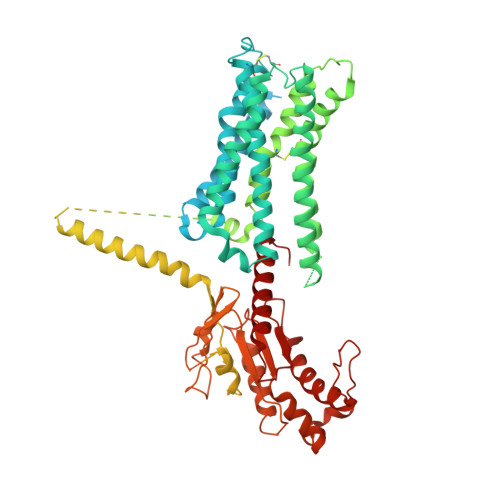
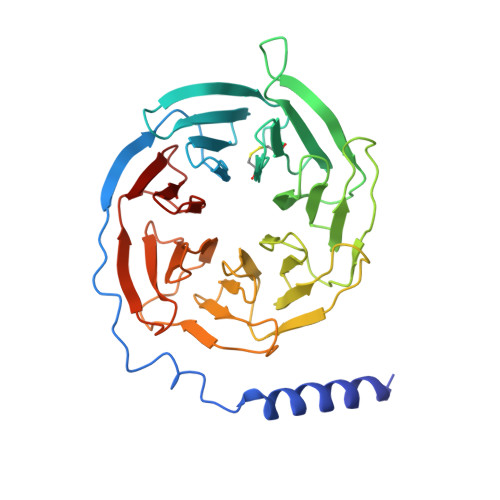
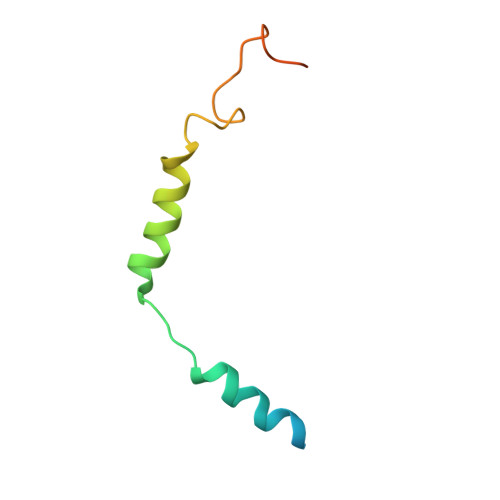
An inverse agonist of orphan receptor GPR61 acts by a G protein-competitive allosteric mechanism.
Lees, J.A., Dias, J.M., Rajamohan, F., Fortin, J.P., O'Connor, R., Kong, J.X., Hughes, E.A.G., Fisher, E.L., Tuttle, J.B., Lovett, G., Kormos, B.L., Unwalla, R.J., Zhang, L., Dechert Schmitt, A.M., Zhou, D., Moran, M., Stevens, K.A., Fennell, K.F., Varghese, A.E., Maxwell, A., Cote, E.E., Zhang, Y., Han, S.(2023) Nat Commun 14: 5938-5938
- PubMed: 37741852
- DOI: https://doi.org/10.1038/s41467-023-41646-3
- Primary Citation of Related Structures:
8TB0, 8TB7 - PubMed Abstract:
GPR61 is an orphan GPCR related to biogenic amine receptors. Its association with phenotypes relating to appetite makes it of interest as a druggable target to treat disorders of metabolism and body weight, such as obesity and cachexia. To date, the lack of structural information or a known biological ligand or tool compound has hindered comprehensive efforts to study GPR61 structure and function. Here, we report a structural characterization of GPR61, in both its active-like complex with heterotrimeric G protein and in its inactive state. Moreover, we report the discovery of a potent and selective small-molecule inverse agonist against GPR61 and structural elucidation of its allosteric binding site and mode of action. These findings offer mechanistic insights into an orphan GPCR while providing both a structural framework and tool compound to support further studies of GPR61 function and modulation.
Organizational Affiliation:
Discovery Sciences, Medicine Design, Pfizer Inc., Groton, CT, USA.