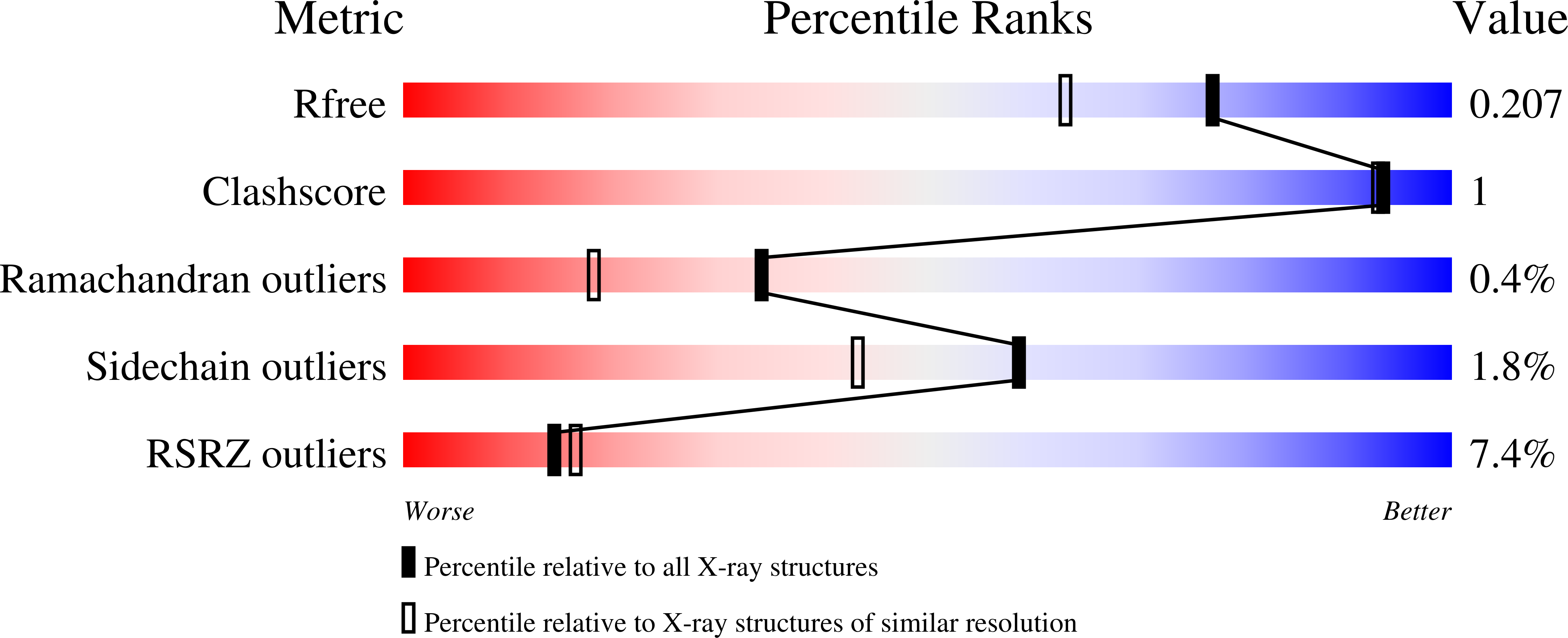
XFEL structure of carbonic anhydrase II: a comparative study of XFEL, NMR, X-ray and neutron structures.
Hull, J.A., Lee, C., Kim, J.K., Lim, S.W., Park, J., Park, S., Lee, S.J., Park, G., Eom, I., Kim, M., Hyun, H., Combs, J.E., Andring, J.T., Lomelino, C., Kim, C.U., McKenna, R.(2024) Acta Crystallogr D Struct Biol 80: 194-202
- PubMed: 38411550
- DOI: https://doi.org/10.1107/S2059798324000482
- Primary Citation of Related Structures:
8SD1, 8SD6, 8SD7, 8SD8, 8SD9, 8SF1 - PubMed Abstract:
The combination of X-ray free-electron lasers (XFELs) with serial femtosecond crystallography represents cutting-edge technology in structural biology, allowing the study of enzyme reactions and dynamics in real time through the generation of `molecular movies'. This technology combines short and precise high-energy X-ray exposure to a stream of protein microcrystals. Here, the XFEL structure of carbonic anhydrase II, a ubiquitous enzyme responsible for the interconversion of CO 2 and bicarbonate, is reported, and is compared with previously reported NMR and synchrotron X-ray and neutron single-crystal structures.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, College of Medicine, University of Florida, Gainesville, FL 32610, USA.