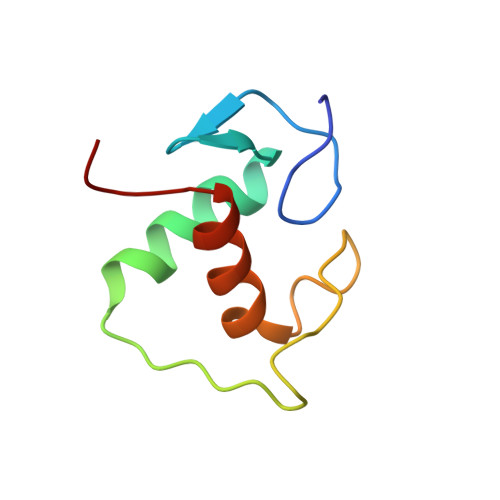
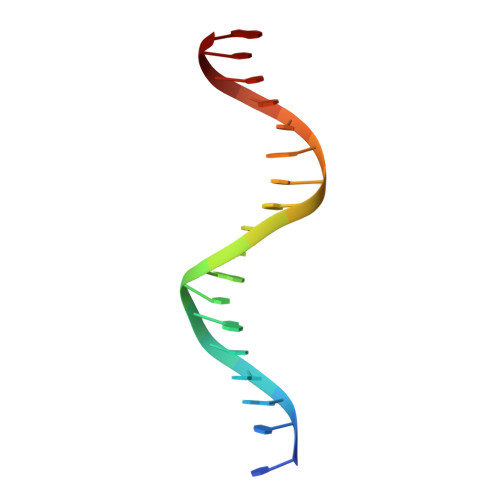
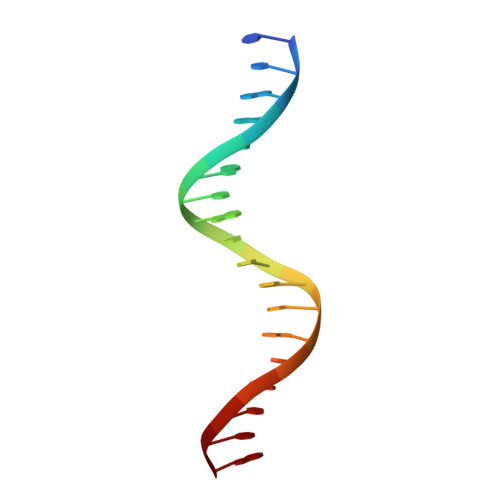
Structural mechanism underlying variations in DNA binding by the androgen receptor.
Lee, X.Y., Van Eynde, W., Helsen, C., Willems, H., Peperstraete, K., De Block, S., Voet, A., Claessens, F.(2024) J Steroid Biochem Mol Biol 241: 106499-106499
- PubMed: 38604378
- DOI: https://doi.org/10.1016/j.jsbmb.2024.106499
- Primary Citation of Related Structures:
8RM6, 8RM7 - PubMed Abstract:
The androgen receptor (AR) is a steroid activated transcription factor which recognizes DNA motifs resembling inverted repeats of a conserved 5'-AGAACA-3'-like hexanucleotides separated by a three-nucleotide spacer from a similar, but less conserved hexanucleotide. Here, we report the structures of the human AR DNA binding domain (DBD) bound to two natural AREs (C3 and MTV) in head-to-head dimer conformations, diffracting at 2.05 Å and 2.25 Å, respectively. These structures help to explain the impact of androgen insensitivity mutations on the structure integrity, DNA binding and DBD dimerization. The binding affinity of the AR DBD to different DNA motifs were measured by the BioLayer Interferometry (BLI) and further validated by Molecular Dynamics (MD) simulations. This shows that the high binding affinity of the first DBD to the upstream 5'-AGAACA-3' motif induces the cooperative binding of the second DBD to the second hexanucleotide. Our data indicate identical interaction of the DBDs to the upstream hexanucleotides, while forming an induced closer contact of the second DBD on the non-canonical hexanucleotides. The variation in binding between the DBD monomers are the result of differences in DNA occupancy, protein-protein interactions, DNA binding affinity, and DNA binding energy profiles. We propose this has functional consequences.
Organizational Affiliation:
Molecular Endocrinology Laboratory, Department of Cellular and Molecular Medicine, Campus Gasthuisberg ON1 Herestraat 49 - box 901, Leuven 3000, Belgium.