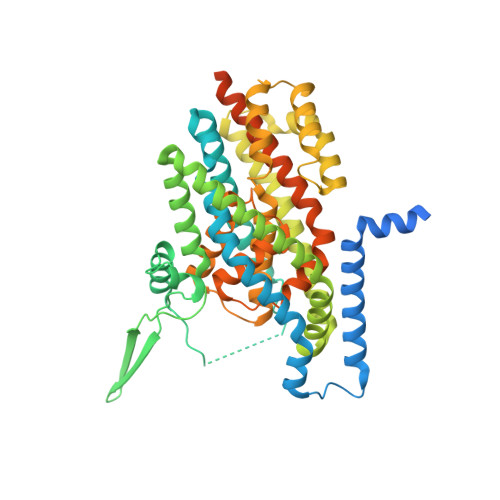
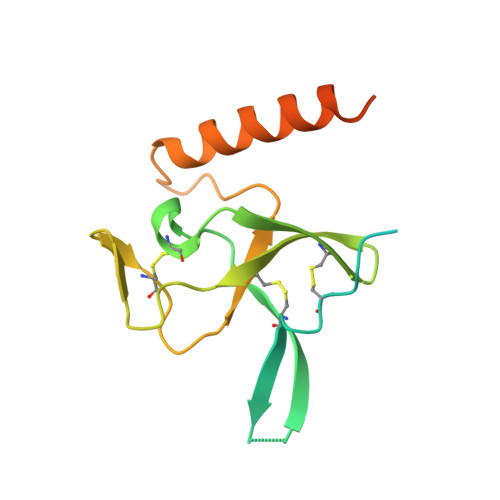
Receptor-recognition and antiviral mechanisms of retrovirus-derived human proteins.
Khare, S., Villalba, M.I., Canul-Tec, J.C., Cajiao, A.B., Kumar, A., Backovic, M., Rey, F.A., Pardon, E., Steyaert, J., Perez, C., Reyes, N.(2024) Nat Struct Mol Biol 31: 1368-1376
- PubMed: 38671230
- DOI: https://doi.org/10.1038/s41594-024-01295-6
- Primary Citation of Related Structures:
8OUD, 8OUH, 8OUI, 8OUJ - PubMed Abstract:
Human syncytin-1 and suppressyn are cellular proteins of retroviral origin involved in cell-cell fusion events to establish the maternal-fetal interface in the placenta. In cell culture, they restrict infections from members of the largest interference group of vertebrate retroviruses, and are regarded as host immunity factors expressed during development. At the core of the syncytin-1 and suppressyn functions are poorly understood mechanisms to recognize a common cellular receptor, the membrane transporter ASCT2. Here, we present cryo-electron microscopy structures of human ASCT2 in complexes with the receptor-binding domains of syncytin-1 and suppressyn. Despite their evolutionary divergence, the two placental proteins occupy similar positions in ASCT2, and are stabilized by the formation of a hybrid β-sheet or 'clamp' with the receptor. Structural predictions of the receptor-binding domains of extant retroviruses indicate overlapping binding interfaces and clamping sites with ASCT2, revealing a competition mechanism between the placental proteins and the retroviruses. Our work uncovers a common ASCT2 recognition mechanism by a large group of endogenous and disease-causing retroviruses, and provides high-resolution views on how placental human proteins exert morphological and immunological functions.
Organizational Affiliation:
Fundamental Microbiology and Pathogenicity Unit, CNRS, Université de Bordeaux, IECB, Bordeaux, France.