Structural insights into the molecular mechanism of high-level ceftazidime-avibactam resistance conferred by CMY-185.
Kawai, A., Shropshire, W.C., Suzuki, M., Borjan, J., Aitken, S.L., Bachman, W.C., McElheny, C.L., Bhatti, M.M., Shields, R.K., Shelburne, S.A., Doi, Y.(2024) mBio 15: e0287423-e0287423
- PubMed: 38179965
- DOI: https://doi.org/10.1128/mbio.02874-23
- Primary Citation of Related Structures:
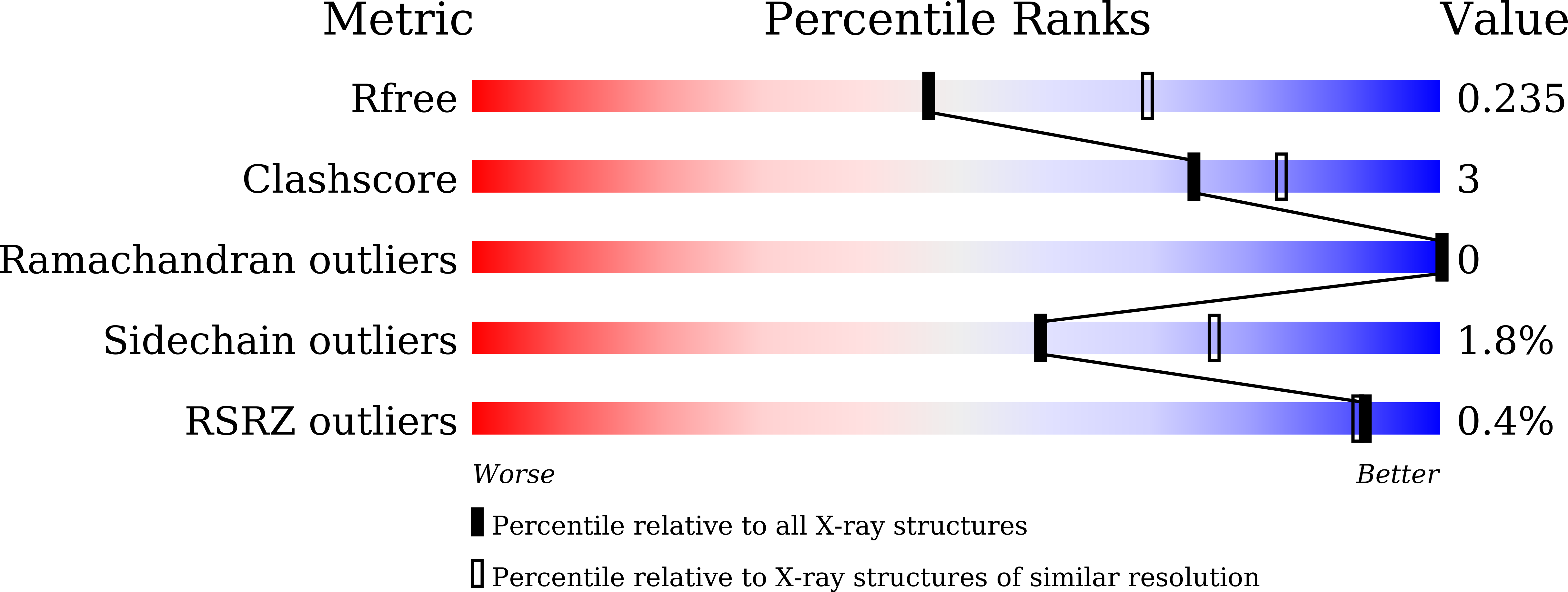
8JB7, 8JB8 - PubMed Abstract:
β-Lactamases can accumulate stepwise mutations that increase their resistance profiles to the latest β-lactam agents. CMY-185 is a CMY-2-like β-lactamase and was identified in an Escherichia coli clinical strain isolated from a patient who underwent treatment with ceftazidime-avibactam. CMY-185, possessing four amino acid substitutions of A114E, Q120K, V211S, and N346Y relative to CMY-2, confers high-level ceftazidime-avibactam resistance, and accumulation of the substitutions incrementally enhances the level of resistance to this agent. However, the functional role of each substitution and their interplay in enabling ceftazidime-avibactam resistance remains unknown. Through biochemical and structural analysis, we present the molecular basis for the enhanced ceftazidime hydrolysis and impaired avibactam inhibition conferred by CMY-185. The substituted Y346 residue is a major driver of the functional evolution as it rejects primary avibactam binding due to the steric hindrance and augments oxyimino-cephalosporin hydrolysis through a drastic structural change, rotating the side chain of Y346 and then disrupting the H-10 helix structure. The other substituted residues E114 and K120 incrementally contribute to rejection of avibactam inhibition, while S211 stimulates the turnover rate of the oxyimino-cephalosporin hydrolysis. These findings indicate that the N346Y substitution is capable of simultaneously expanding the spectrum of activity against some of the latest β-lactam agents with altered bulky side chains and rejecting the binding of β-lactamase inhibitors. However, substitution of additional residues may be required for CMY enzymes to achieve enhanced affinity or turnover rate of the β-lactam agents leading to clinically relevant levels of resistance.IMPORTANCECeftazidime-avibactam has a broad spectrum of activity against multidrug-resistant Gram-negative bacteria including carbapenem-resistant Enterobacterales including strains with or without production of serine carbapenemases. After its launch, emergence of ceftazidime-avibactam-resistant strains that produce mutated β-lactamases capable of efficiently hydrolyzing ceftazidime or impairing avibactam inhibition are increasingly reported. Furthermore, cross-resistance towards cefiderocol, the latest cephalosporin in clinical use, has been observed in some instances. Here, we clearly demonstrate the functional role of the substituted residues in CMY-185, a four amino-acid variant of CMY-2 identified in a patient treated with ceftazidime-avibactam, for high-level resistance to this agent and low-level resistance to cefiderocol. These findings provide structural insights into how β-lactamases may incrementally alter their structures to escape multiple advanced β-lactam agents.
Organizational Affiliation:
Department of Microbiology, Fujita Health University School of Medicine, Toyoake, Aichi, Japan.