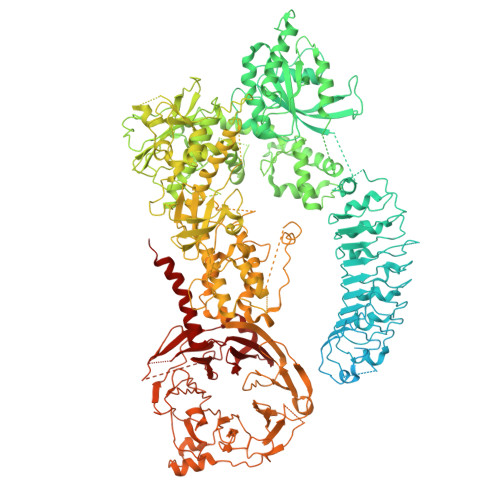
Structure and regulation of full-length human leucine-rich repeat kinase 1.
Metcalfe, R.D., Martinez Fiesco, J.A., Bonet-Ponce, L., Kluss, J.H., Cookson, M.R., Zhang, P.(2023) Nat Commun 14: 4797-4797
- PubMed: 37558661
- DOI: https://doi.org/10.1038/s41467-023-40532-2
- Primary Citation of Related Structures:
8FAC - PubMed Abstract:
The human leucine-rich repeat kinases (LRRKs), LRRK1 and LRRK2 are large and unusually complex multi-domain kinases, which regulate fundamental cellular processes and have been implicated in human disease. Structures of LRRK2 have recently been determined, but the structure and molecular mechanisms regulating the activity of the LRRK1 as well as differences in the regulation of LRRK1 and LRRK2 remain unclear. Here, we report a cryo-EM structure of the LRRK1 monomer and a lower-resolution cryo-EM map of the LRRK1 dimer. The monomer structure, in which the kinase is in an inactive conformation, reveals key interdomain interfaces that control kinase activity as we validate experimentally. Both the LRRK1 monomer and dimer are structurally distinct compared to LRRK2. Overall, our results provide structural insights into the activation of the human LRRKs, which advance our understanding of their physiological and pathological roles.
Organizational Affiliation:
Center for Structural Biology, Center for Cancer Research, National Cancer Institute, Frederick, MD, 21702, USA.