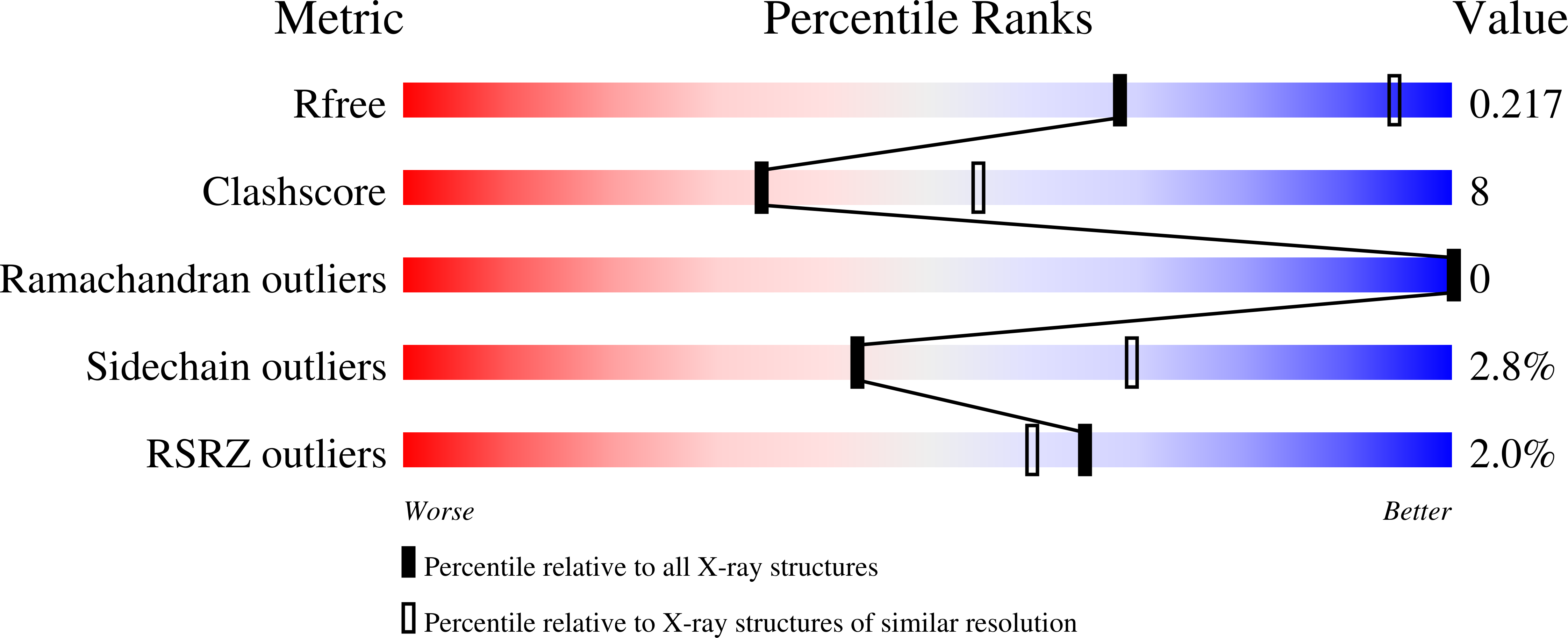
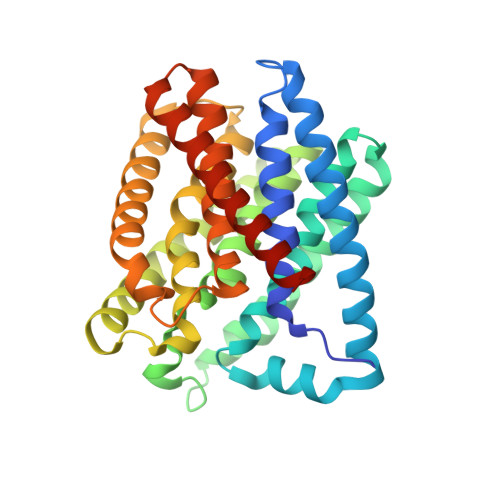
Multi-crystal native-SAD phasing at 5 keV with a helium environment.
Karasawa, A., Andi, B., Fuchs, M.R., Shi, W., McSweeney, S., Hendrickson, W.A., Liu, Q.(2022) IUCrJ 9: 768-777
- PubMed: 36381147
- DOI: https://doi.org/10.1107/S205225252200971X
- Primary Citation of Related Structures:
8EN9, 8ENA - PubMed Abstract:
De novo structure determination from single-wavelength anomalous diffraction using native sulfur or phospho-rus in biomolecules (native-SAD) is an appealing method to mitigate the labor-intensive production of heavy-atom derivatives and seleno-methio-nyl substitutions. The native-SAD method is particularly attractive for membrane proteins, which are difficult to produce and often recalcitrant to grow into decent-sized crystals. Native-SAD uses lower-energy X-rays to enhance anomalous signals from sulfur or phospho-rus. However, at lower energies, the scattering and absorption of air contribute to the background noise, reduce the signals and are thus adverse to native-SAD phasing. We have previously demonstrated native-SAD phasing at an energy of 5 keV in air at the NSLS-II FMX beamline. Here, the use of a helium path developed to reduce both the noise from background scattering and the air absorption of the diffracted X-ray beam are described. The helium path was used for collection of anomalous diffraction data at 5 keV for two proteins: thaumatin and the membrane protein TehA. Although anomalous signals from each individual crystal are very weak, robust anomalous signals are obtained from data assembled from micrometre-sized crystals. The thaumatin structure was determined from 15 microcrystals and the TehA structure from 18 microcrystals. These results demonstrate the usefulness of a helium environment in support of native-SAD phasing at 5 keV.
Organizational Affiliation:
Center on Membrane Protein Production and Analysis, New York Structural Biology Center, New York, NY 10027, USA.