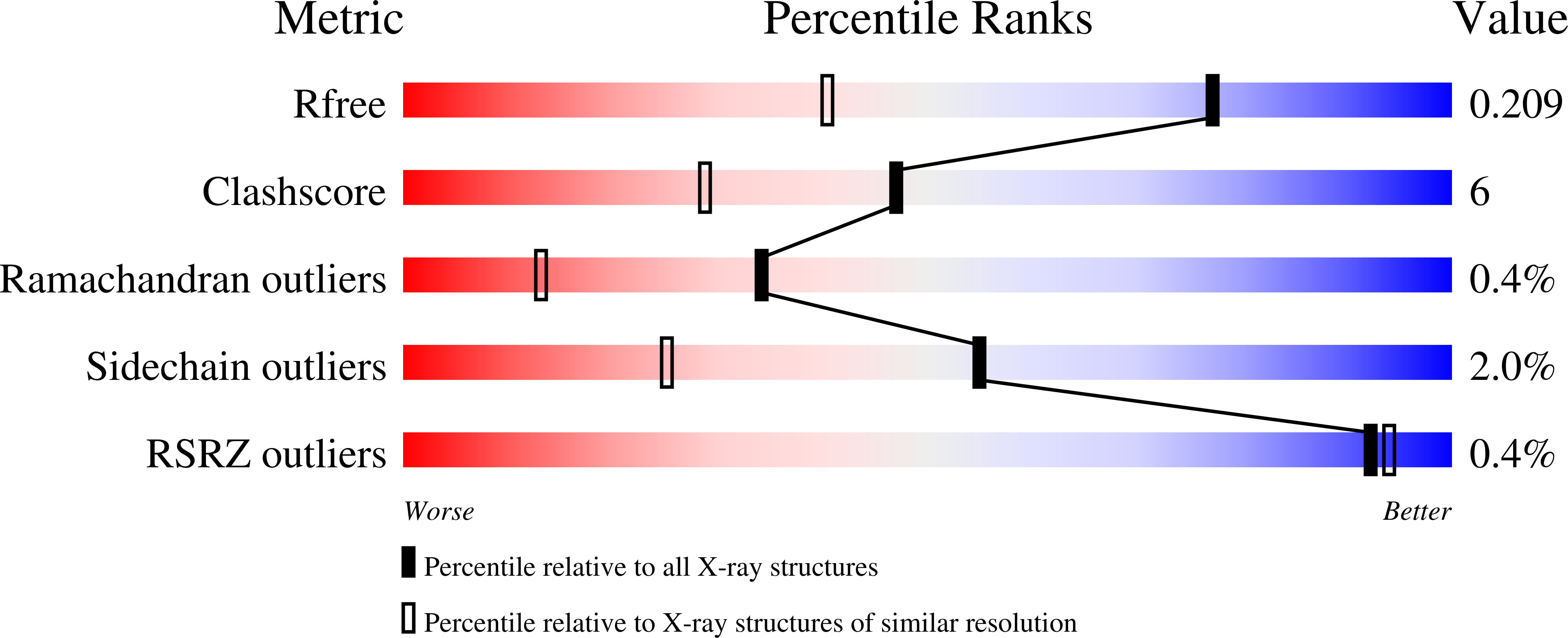
Mutagenesis and structural analysis reveal the CTX-M beta-lactamase active site is optimized for cephalosporin catalysis and drug resistance.
Lu, S., Montoya, M., Hu, L., Neetu, N., Sankaran, B., Prasad, B.V.V., Palzkill, T.(2023) J Biol Chem 299: 104630-104630
- PubMed: 36963495
- DOI: https://doi.org/10.1016/j.jbc.2023.104630
- Primary Citation of Related Structures:
8DOD, 8DOE, 8DON, 8DP4, 8DPQ, 8ELA, 8ELB - PubMed Abstract:
CTX-M β-lactamases are a widespread source of resistance to β-lactam antibiotics in Gram-negative bacteria. These enzymes readily hydrolyze penicillins and cephalosporins, including oxyimino-cephalosporins such as cefotaxime. To investigate the preference of CTX-M enzymes for cephalosporins, we examined eleven active-site residues in the CTX-M-14 β-lactamase model system by alanine mutagenesis to assess the contribution of the residues to catalysis and specificity for the hydrolysis of the penicillin, ampicillin, and the cephalosporins cephalothin and cefotaxime. Key active site residues for class A β-lactamases, including Lys73, Ser130, Asn132, Lys234, Thr216, and Thr235, contribute significantly to substrate binding and catalysis of penicillin and cephalosporin substrates in that alanine substitutions decrease both k cat and k cat /K M . A second group of residues, including Asn104, Tyr105, Asn106, Thr215, and Thr216, contribute only to substrate binding, with the substitutions decreasing only k cat /K M . Importantly, calculating the average effect of a substitution across the 11 active-site residues shows that the most significant impact is on cefotaxime hydrolysis while ampicillin hydrolysis is least affected, suggesting the active site is highly optimized for cefotaxime catalysis. Furthermore, we determined X-ray crystal structures for the apo-enzymes of the mutants N106A, S130A, N132A, N170A, T215A, and T235A. Surprisingly, in the structures of some mutants, particularly N106A and T235A, the changes in structure propagate from the site of substitution to other regions of the active site, suggesting that the impact of substitutions is due to more widespread changes in structure and illustrating the interconnected nature of the active site.
Organizational Affiliation:
Department of Pharmacology and Chemical Biology, Baylor College of Medicine, Houston, Texas, USA.