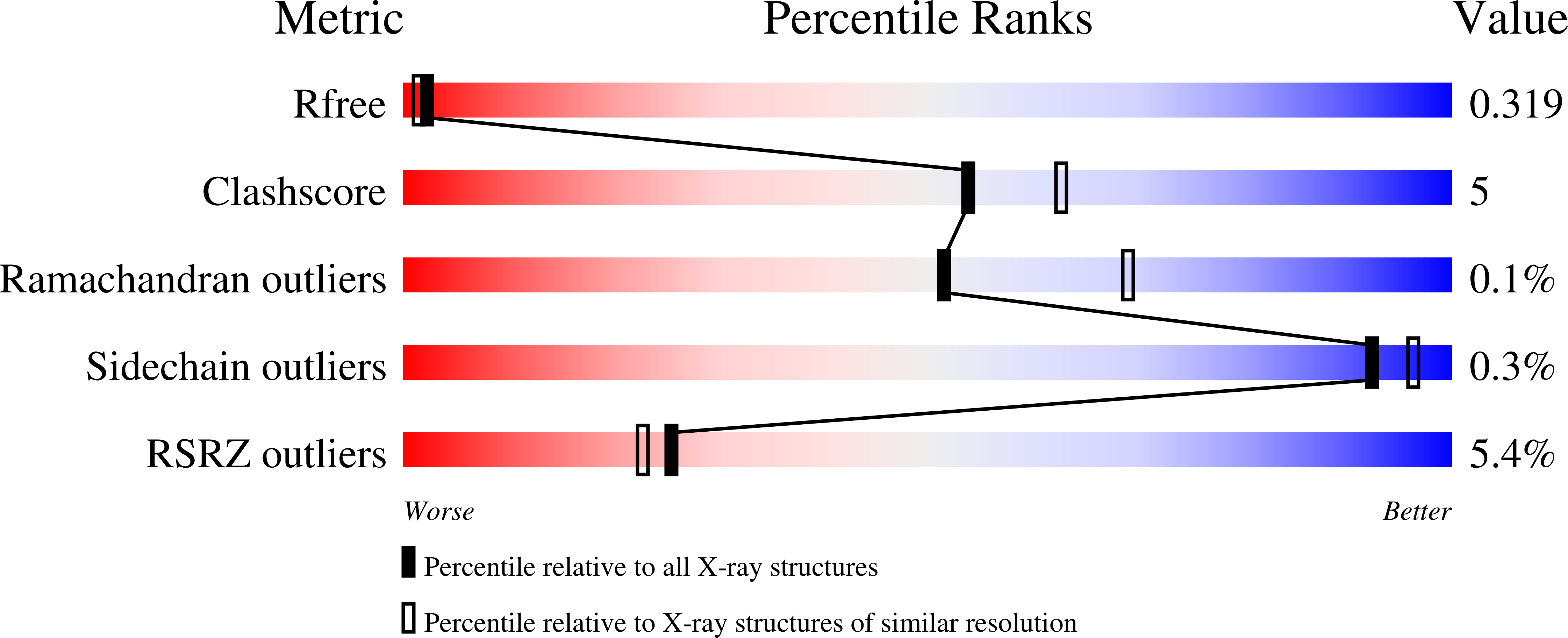
Characterization and structure of the human lysine-2-oxoglutarate reductase domain, a novel therapeutic target for treatment of glutaric aciduria type 1.
Leandro, J., Khamrui, S., Suebsuwong, C., Chen, P.J., Secor, C., Dodatko, T., Yu, C., Sanchez, R., DeVita, R.J., Houten, S.M., Lazarus, M.B.(2022) Open Biol 12: 220179-220179
- PubMed: 36128717
- DOI: https://doi.org/10.1098/rsob.220179
- Primary Citation of Related Structures:
8E8T, 8E8U, 8E8V - PubMed Abstract:
In humans, a single enzyme 2-aminoadipic semialdehyde synthase (AASS) catalyses the initial two critical reactions in the lysine degradation pathway. This enzyme evolved to be a bifunctional enzyme with both lysine-2-oxoglutarate reductase (LOR) and saccharopine dehydrogenase domains (SDH). Moreover, AASS is a unique drug target for inborn errors of metabolism such as glutaric aciduria type 1 that arise from deficiencies downstream in the lysine degradation pathway. While work has been done to elucidate the SDH domain structurally and to develop inhibitors, neither has been done for the LOR domain. Here, we purify and characterize LOR and show that it is activated by alkylation of cysteine 414 by N-ethylmaleimide. We also provide evidence that AASS is rate-limiting upon high lysine exposure of mice. Finally, we present the crystal structure of the human LOR domain. Our combined work should enable future efforts to identify inhibitors of this novel drug target.
Organizational Affiliation:
Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA.