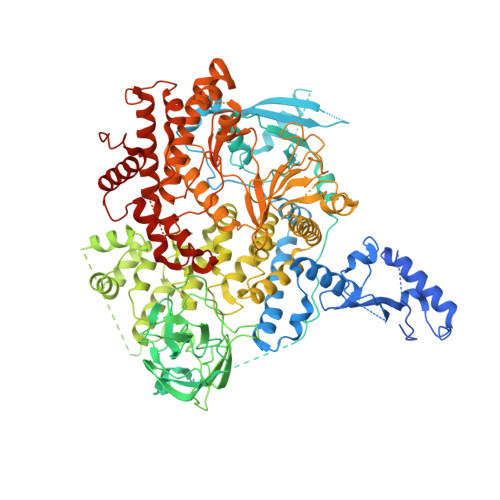
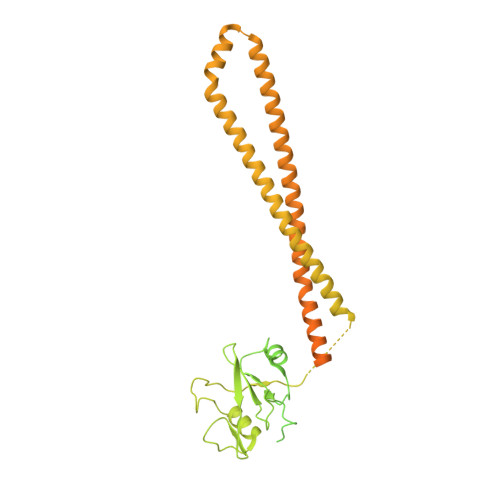
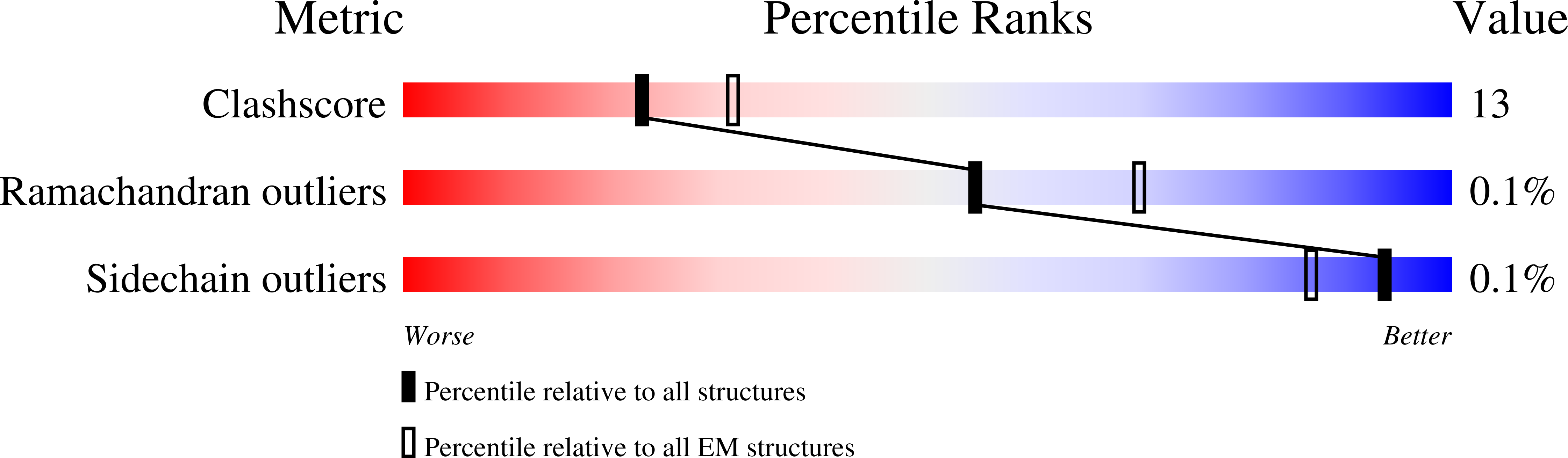Nanobodies and chemical cross-links advance the structural and functional analysis of PI3K alpha.
Hart, J.R., Liu, X., Pan, C., Liang, A., Ueno, L., Xu, Y., Quezada, A., Zou, X., Yang, S., Zhou, Q., Schoonooghe, S., Hassanzadeh-Ghassabeh, G., Xia, T., Shui, W., Yang, D., Vogt, P.K., Wang, M.W.(2022) Proc Natl Acad Sci U S A 119: e2210769119-e2210769119
- PubMed: 36095215
- DOI: https://doi.org/10.1073/pnas.2210769119
- Primary Citation of Related Structures:
8DCP, 8DCX, 8DD4, 8DD8 - PubMed Abstract:
Nanobodies and chemical cross-linking were used to gain information on the identity and positions of flexible domains of PI3Kα. The application of chemical cross-linking mass spectrometry (CXMS) facilitated the identification of the p85 domains BH, cSH2, and SH3 as well as their docking positions on the PI3Kα catalytic core. Binding of individual nanobodies to PI3Kα induced activation or inhibition of enzyme activity and caused conformational changes that could be correlated with enzyme function. Binding of nanobody Nb3-126 to the BH domain of p85α substantially improved resolution for parts of the PI3Kα complex, and binding of nanobody Nb3-159 induced a conformation of PI3Kα that is distinct from known PI3Kα structures. The analysis of CXMS data also provided mechanistic insights into the molecular underpinning of the flexibility of PI3Kα.
Organizational Affiliation:
Department of Molecular Medicine, Scripps Research Institute, La Jolla, CA 92037.