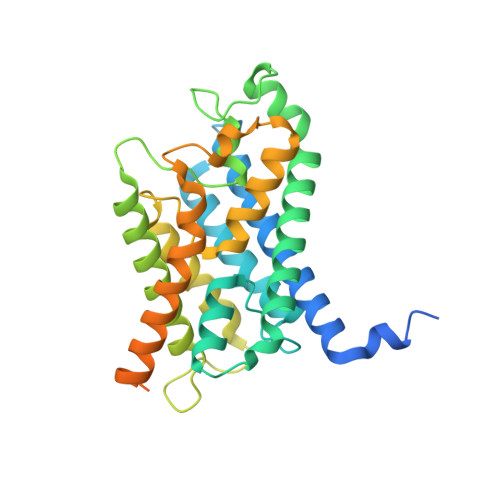
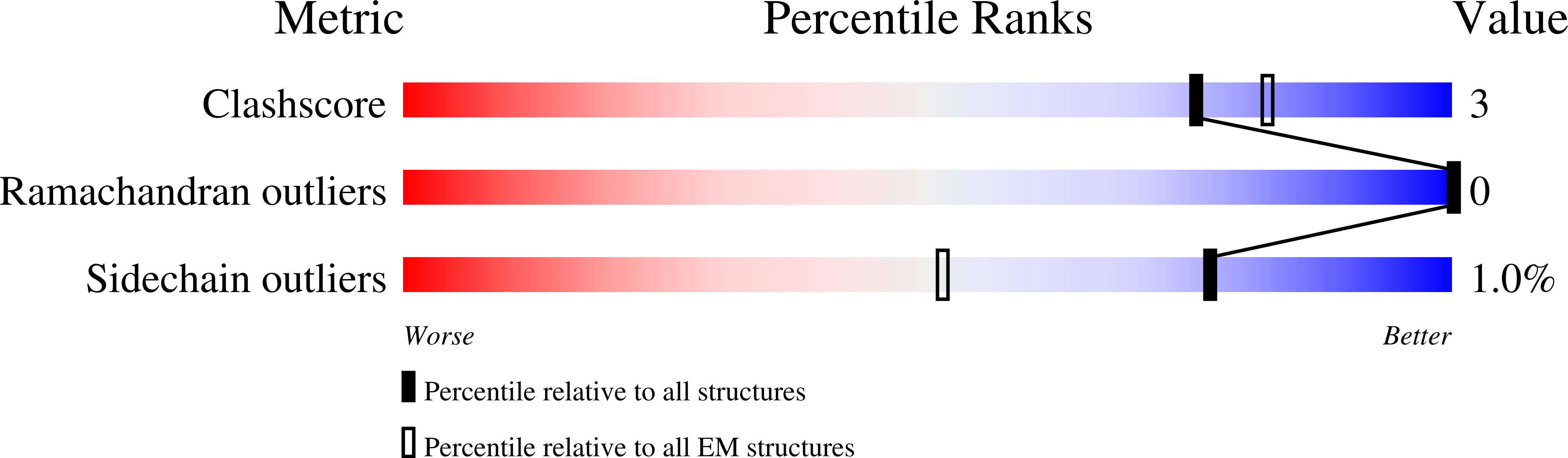Cryo-EM structure supports a role of AQP7 as a junction protein.
Huang, P., Venskutonyte, R., Prasad, R.B., Ardalani, H., de Mare, S.W., Fan, X., Li, P., Spegel, P., Yan, N., Gourdon, P., Artner, I., Lindkvist-Petersson, K.(2023) Nat Commun 14: 600-600
- PubMed: 36737436
- DOI: https://doi.org/10.1038/s41467-023-36272-y
- Primary Citation of Related Structures:
8AMW, 8AMX - PubMed Abstract:
Aquaglyceroporin 7 (AQP7) facilitates glycerol flux across the plasma membrane with a critical physiological role linked to metabolism, obesity, and associated diseases. Here, we present the single-particle cryo-EM structure of AQP7 determined at 2.55 Å resolution adopting two adhering tetramers, stabilized by extracellularly exposed loops, in a configuration like that of the well-characterized interaction of AQP0 tetramers. The central pore, in-between the four monomers, displays well-defined densities restricted by two leucine filters. Gas chromatography mass spectrometry (GC/MS) results show that the AQP7 sample contains glycerol 3-phosphate (Gro3P), which is compatible with the identified features in the central pore. AQP7 is shown to be highly expressed in human pancreatic α- and β- cells suggesting that the identified AQP7 octamer assembly, in addition to its function as glycerol channel, may serve as junction proteins within the endocrine pancreas.
Organizational Affiliation:
Department of Experimental Medical Science, Lund University, Lund, Sweden.