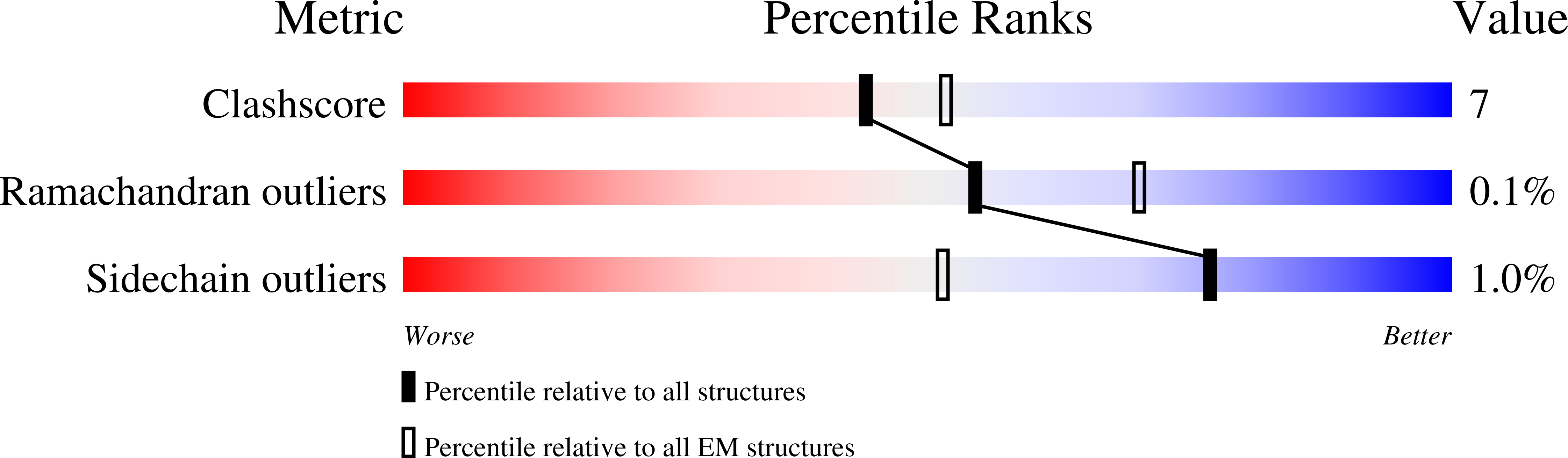Outer membrane utilisomes mediate glycan uptake in gut Bacteroidetes.
White, J.B.R., Silale, A., Feasey, M., Heunis, T., Zhu, Y., Zheng, H., Gajbhiye, A., Firbank, S., Basle, A., Trost, M., Bolam, D.N., van den Berg, B., Ranson, N.A.(2023) Nature 618: 583-589
- PubMed: 37286596
- DOI: https://doi.org/10.1038/s41586-023-06146-w
- Primary Citation of Related Structures:
7ZNR, 7ZNS, 8A9Y, 8AA0, 8AA1, 8AA2, 8AA3, 8AA4 - PubMed Abstract:
Bacteroidetes are abundant members of the human microbiota, utilizing a myriad of diet- and host-derived glycans in the distal gut 1 . Glycan uptake across the bacterial outer membrane of these bacteria is mediated by SusCD protein complexes, comprising a membrane-embedded barrel and a lipoprotein lid, which is thought to open and close to facilitate substrate binding and transport. However, surface-exposed glycan-binding proteins and glycoside hydrolases also play critical roles in the capture, processing and transport of large glycan chains. The interactions between these components in the outer membrane are poorly understood, despite being crucial for nutrient acquisition by our colonic microbiota. Here we show that for both the levan and dextran utilization systems of Bacteroides thetaiotaomicron, the additional outer membrane components assemble on the core SusCD transporter, forming stable glycan-utilizing machines that we term utilisomes. Single-particle cryogenic electron microscopy structures in the absence and presence of substrate reveal concerted conformational changes that demonstrate the mechanism of substrate capture, and rationalize the role of each component in the utilisome.
Organizational Affiliation:
Astbury Centre for Structural Molecular Biology, Faculty of Biological Sciences, University of Leeds, Leeds, UK.