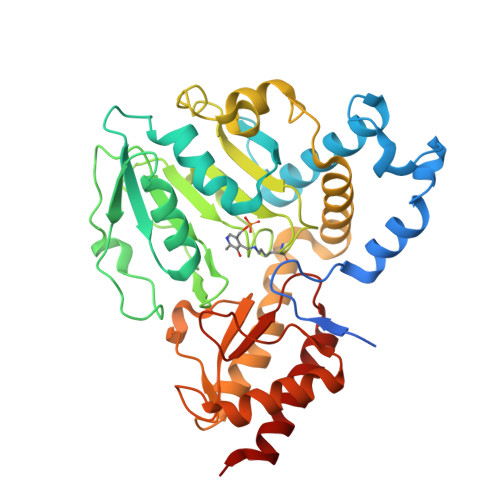
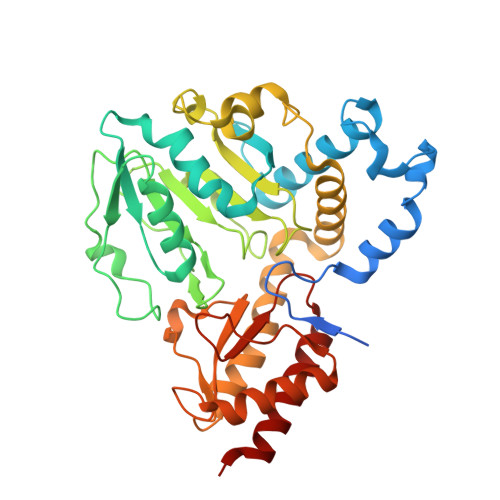
L-serine biosynthesis in the human central nervous system: Structure and function of phosphoserine aminotransferase.
Marchesani, F., Zangelmi, E., Murtas, G., Costanzi, E., Ullah, R., Peracchi, A., Bruno, S., Pollegioni, L., Mozzarelli, A., Storici, P., Campanini, B.(2023) Protein Sci 32: e4609-e4609
- PubMed: 36851825
- DOI: https://doi.org/10.1002/pro.4609
- Primary Citation of Related Structures:
8A5V, 8A5W - PubMed Abstract:
Organisms from all kingdoms of life synthesize L-serine (L-Ser) from 3-phosphoglycerate through the phosphorylated pathway, a three-step diversion of glycolysis. Phosphoserine aminotransferase (PSAT) catalyzes the intermediate step, the pyridoxal 5'-phosphate-dependent transamination of 3-phosphohydroxypyruvate and L-glutamate to O-phosphoserine (OPS) and α-ketoglutarate. PSAT is particularly relevant in the central nervous system of mammals because L-Ser is the metabolic precursor of D-serine, cysteine, phospholipids, and nucleotides. Several mutations in the human psat gene have been linked to serine deficiency disorders, characterized by severe neurological symptoms. Furthermore, PSAT is overexpressed in many tumors and this overexpression has been associated with poor clinical outcomes. Here, we report the detailed functional and structural characterization of the recombinant human PSAT. The reaction catalyzed by PSAT is reversible, with an equilibrium constant of about 10, and the enzyme is very efficient, with a k cat /K m of 5.9 × 10 6 M -1 s -1 , thus contributing in driving the pathway towards the products despite the extremely unfavorable first step catalyzed by 3-phosphoglycerate dehydrogenase. The 3D X-ray crystal structure of PSAT was solved in the substrate-free as well as in the OPS-bound forms. Both structures contain eight protein molecules in the asymmetric unit, arranged in four dimers, with a bound cofactor in each subunit. In the substrate-free form, the active site of PSAT contains a sulfate ion that, in the substrate-bound form, is replaced by the phosphate group of OPS. Interestingly, fast crystal soaking used to produce the substrate-bound form allowed the trapping of different intermediates along the catalytic cycle.
Organizational Affiliation:
Department of Food and Drug, University of Parma, Parma, Italy.