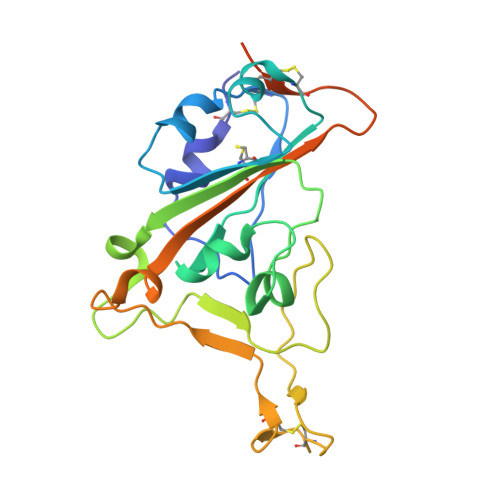
Structural Basis for Evasion of New SARS-CoV-2 Variants from the Potent Virus-Neutralizing Nanobody Targeting the S-Protein Receptor-Binding Domain.
Sluchanko, N.N., Shcheblyakov, D.V., Varfolomeeva, L.A., Favorskaya, I.A., Dolzhikova, I.V., Korobkova, A.I., Alekseeva, I.A., Esmagambetov, I.B., Derkaev, A.A., Prokofiev, V.V., Zorkov, I.D., Logunov, D.Y., Gintsburg, A.L., Popov, V.O., Boyko, K.M.(2024) Biochemistry (Mosc) 89: 1260-1272
- PubMed: 39218023
- DOI: https://doi.org/10.1134/S0006297924070083
- Primary Citation of Related Structures:
8ZER, 8ZES - PubMed Abstract:
COVID-19 has caused millions of deaths and many times more infections worldwide, emphasizing the unpreparedness of the global health system in the face of new infections and the key role for vaccines and therapeutics, including virus-neutralizing antibodies, in prevention and containment of the disease. Continuous evolution of the SARS-CoV-2 coronavirus has been causing its new variants to evade the action of the immune system, which highlighted the importance of detailed knowledge of the epitopes of already selected potent virus-neutralizing antibodies. A single-chain antibody ("nanobody") targeting the SARS-CoV-2 receptor-binding domain (RBD), clone P2C5, had exhibited robust virus-neutralizing activity against all SARS-CoV-2 variants and, being a major component of the anti-COVID-19 formulation "GamCoviMab", had successfully passed Phase I of clinical trials. However, after the emergence of the Delta and XBB variants, a decrease in the neutralizing activity of this nanobody was observed. Here we report on the successful crystal structure determination of the RBD:P2C5 complex at 3.1 Å, which revealed the intricate protein-protein interface, sterically occluding full ACE2 receptor binding by the P2C5-neutralized RBD. Moreover, the structure revealed the developed RBD:P2C5 interface centered around residues Leu452 and Phe490, thereby explaining the evasion of the Delta or Omicron XBB, but not Omicron B.1.1.529 variant, as a result of the single L452R or F490S mutations, respectively, from the action of P2C5. The structure obtained is expected to foster nanobody engineering in order to rescue neutralization activity and will facilitate epitope mapping for other neutralizing nanobodies by competition assays.
Organizational Affiliation:
Bach Institute of Biochemistry, Federal Research Centre "Fundamentals of Biotechnology", Russian Academy of Sciences, Moscow, 119071, Russia. nikolai.sluchanko@mail.ru.