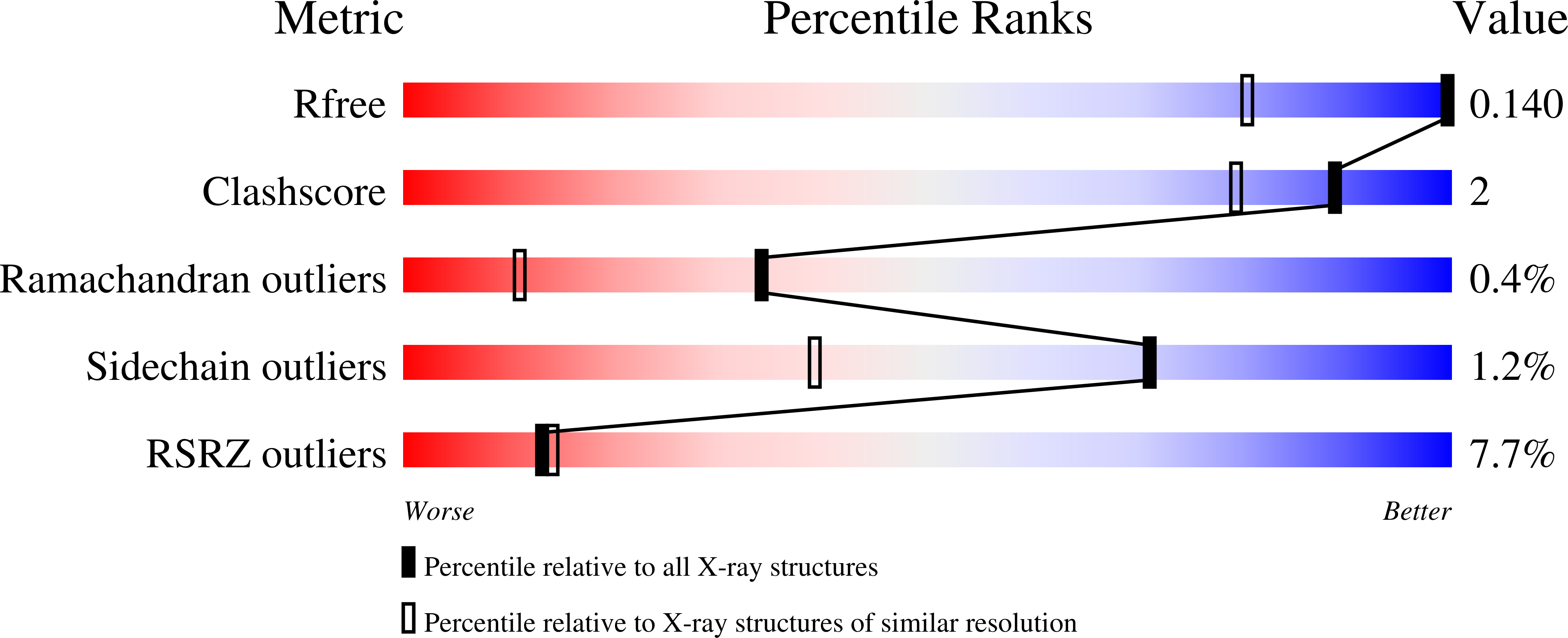
Changes in an Enzyme Ensemble During Catalysis Observed by High Resolution XFEL Crystallography.
Smith, N., Dasgupta, M., Wych, D.C., Dolamore, C., Sierra, R.G., Lisova, S., Marchany-Rivera, D., Cohen, A.E., Boutet, S., Hunter, M.S., Kupitz, C., Poitevin, F., Moss, F.R., Brewster, A.S., Sauter, N.K., Young, I.D., Wolff, A.M., Tiwari, V.K., Kumar, N., Berkowitz, D.B., Hadt, R.G., Thompson, M.C., Follmer, A.H., Wall, M.E., Wilson, M.A.(2023) bioRxiv
- PubMed: 37645800
- DOI: https://doi.org/10.1101/2023.08.15.553460
- Primary Citation of Related Structures:
8TSU, 8TSX, 8TSY, 8TSZ, 8TT0, 8TT1, 8TT2, 8TT4, 8TT5 - PubMed Abstract:
Enzymes populate ensembles of structures with intrinsically different catalytic proficiencies that are difficult to experimentally characterize. We use time-resolved mix-and-inject serial crystallography (MISC) at an X-ray free electron laser (XFEL) to observe catalysis in a designed mutant (G150T) isocyanide hydratase (ICH) enzyme that enhances sampling of important minor conformations. The active site exists in a mixture of conformations and formation of the thioimidate catalytic intermediate selects for catalytically competent substates. A prior proposal for active site cysteine charge-coupled conformational changes in ICH is validated by determining structures of the enzyme over a range of pH values. A combination of large molecular dynamics simulations of the enzyme in crystallo and time-resolved electron density maps shows that ionization of the general acid Asp17 during catalysis causes additional conformational changes that propagate across the dimer interface, connecting the two active sites. These ionization-linked changes in the ICH conformational ensemble permit water to enter the active site in a location that is poised for intermediate hydrolysis. ICH exhibits a tight coupling between ionization of active site residues and catalysis-activated protein motions, exemplifying a mechanism of electrostatic control of enzyme dynamics.
Organizational Affiliation:
Department of Biochemistry and Redox Biology Center, University of Nebraska-Lincoln, Lincoln, NE, 68588.