Structural and biochemical elucidation of class I hybrid cluster protein natively extracted from a marine methanogenic archaeon.
Lemaire, O.N., Belhamri, M., Wagner, T.(2023) Front Microbiol 14: 1179204-1179204
- PubMed: 37250035
- DOI: https://doi.org/10.3389/fmicb.2023.1179204
- Primary Citation of Related Structures:
8CNR, 8CNS - PubMed Abstract:
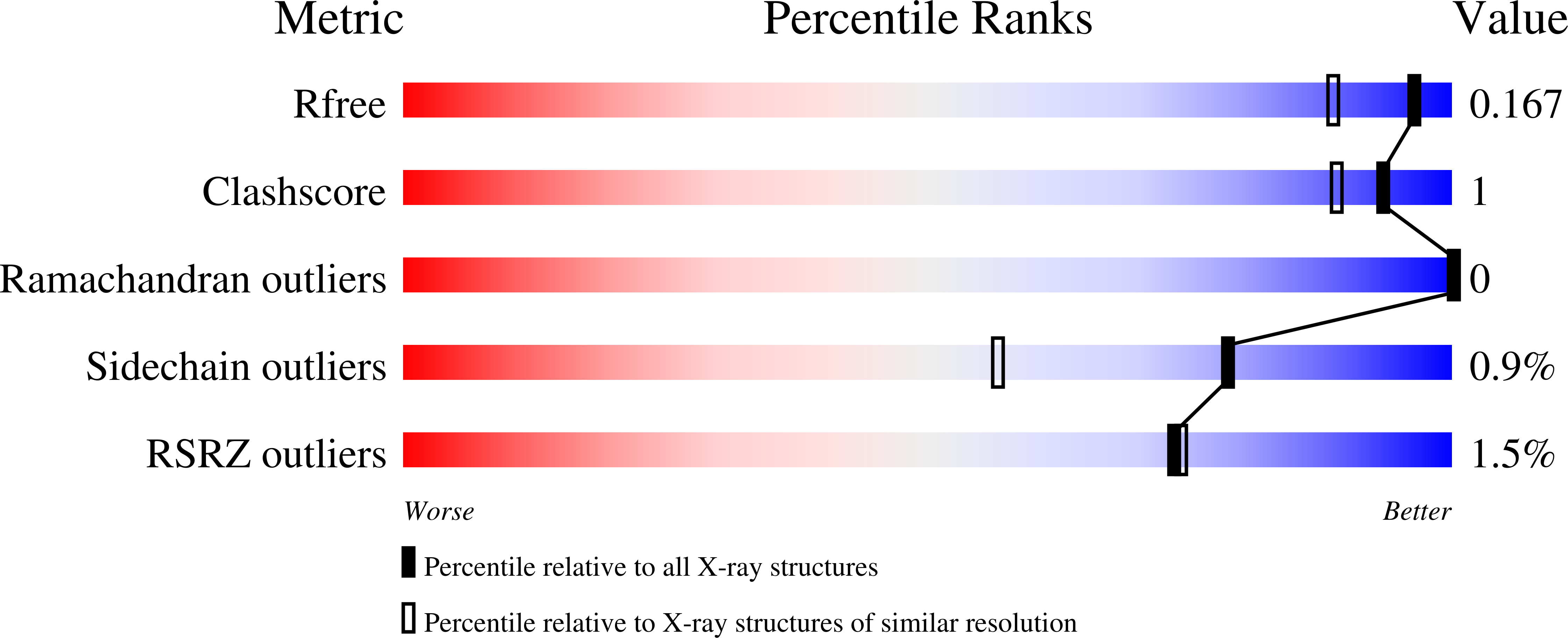
Whilst widespread in the microbial world, the hybrid cluster protein (HCP) has been paradoxically a long-time riddle for microbiologists. During three decades, numerous studies on a few model organisms unravelled its structure and dissected its metal-containing catalyst, but the physiological function of the enzyme remained elusive. Recent studies on bacteria point towards a nitric oxide reductase activity involved in resistance during nitrate and nitrite reduction as well as host infection. In this study, we isolated and characterised a naturally highly produced HCP class I from a marine methanogenic archaeon grown on ammonia. The crystal structures of the enzyme in a reduced and partially oxidised state, obtained at a resolution of 1.45 and 1.36-Å, respectively, offered a precise picture of the archaeal enzyme intimacy. There are striking similarities with the well-studied enzymes from Desulfovibrio species regarding sequence, kinetic parameters, structure, catalyst conformations, and internal channelling systems. The close phylogenetic relationship between the enzymes from Methanococcales and many Bacteria corroborates this similarity. Indeed, Methanococcales HCPs are closer to these bacterial homologues than to any other archaeal enzymes. The relatively high constitutive production of HCP in M. thermolithotrophicus , in the absence of a notable nitric oxide source, questions the physiological function of the enzyme in these ancient anaerobes.
Organizational Affiliation:
Max Planck Institute for Marine Microbiology, Bremen, Germany.