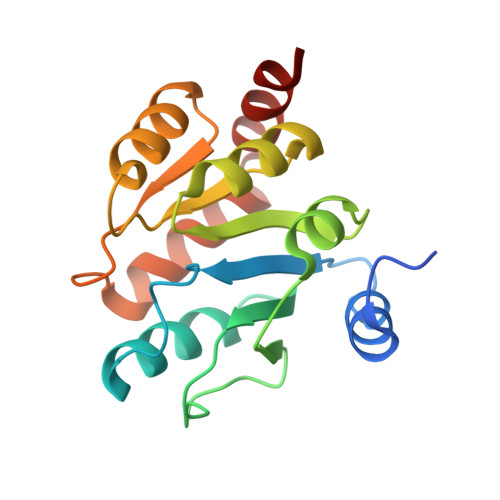
Crystal Structure Determination of Nucleotide-sugar Binding Domain of Human UDP-glucuronosyltransferases 2B10.
Yin, X., Lu, X., Qi, X., Tu, Y., Zhang, N., Yang, Y., Chen, X., Tong, J.(2023) Protein Pept Lett 30: 941-950
- PubMed: 37946357
- DOI: https://doi.org/10.2174/0109298665255492231020050937
- Primary Citation of Related Structures:
7YF5 - PubMed Abstract:
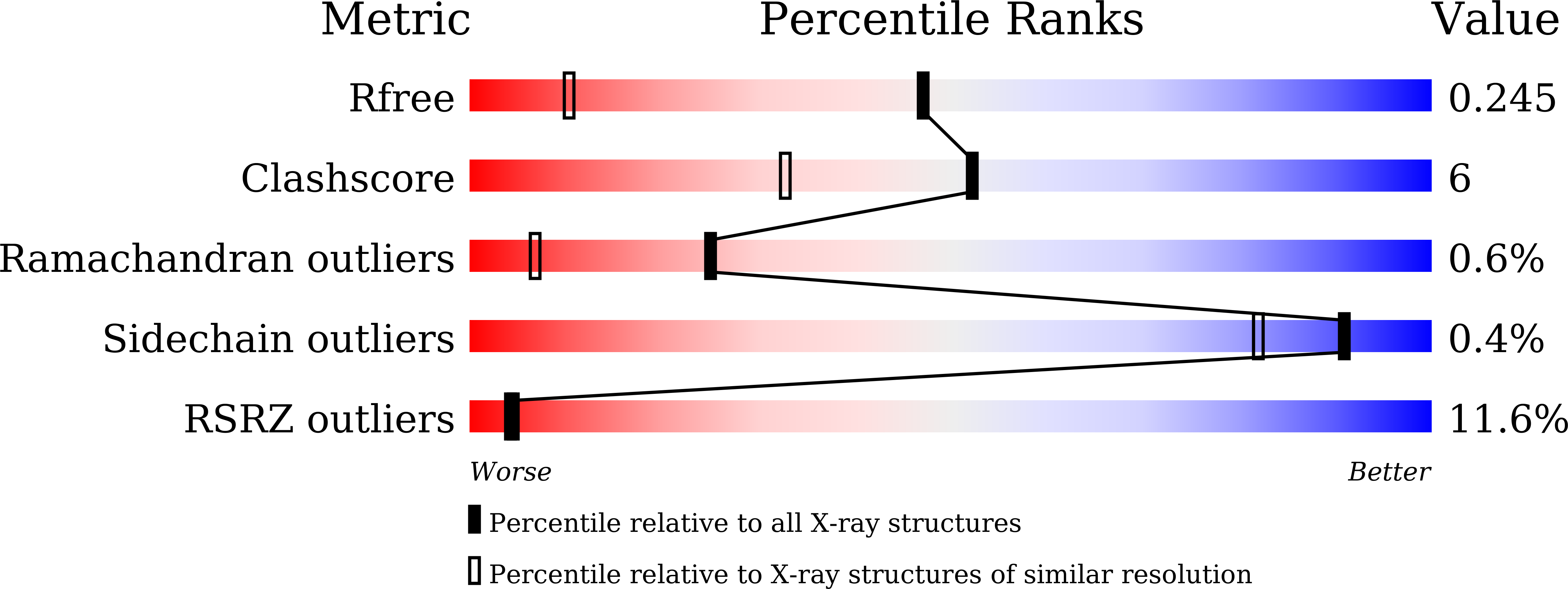
UDP-glucuronosyltransferases (UGTs) play a crucial role in maintaining endobiotic homeostasis and metabolizing xenobiotic compounds, particularly clinical drugs. However, the detailed catalytic mechanism of UGTs has not been fully elucidated due to the limited availability of reliable protein structures. Determining the catalytic domain of human UGTs has proven to be a significant challenge, primarily due to the difficulty in purifying and crystallizing the full-length protein. This study focused on the human UGT2B10 C-terminal cofactor binding domain, aiming to provide structural insights into the fundamental catalytic mechanisms. In this study, the C-terminal sugar-donor binding domain of human UGT2B10 was purified and crystallized using the vapor-diffusion method. The resulting UGT2B10 CTD crystals displayed high-quality diffraction patterns, allowing for data collection at an impressive resolution of 1.53 Å using synchrotron radiation. Subsequently, the structure of the UGT2B10 CTD was determined using the molecule replacement method with a homologous structure. The crystals were monoclinic, belonging to the space C2 with unit-cell parameters a = 85.90 Å, b = 58.39 Å, c = 68.87 Å, α = γ = 90°, and β = 98.138°. The Matthews coefficient V M was determined to be 2.24 Å 3 Da -1 (solvent content 46.43%) with two molecules in the asymmetric unit. The crystal structure of UGT2B10 CTD was solved at a high resolution of 1.53 Å, revealing a conserved cofactor binding pocket. This is the first study determining the C-terminal cofactor binding domain of human UGT2B10, which plays a key role in additive drug metabolism.
Organizational Affiliation:
School of Pharmacy, Hangzhou Normal University, Hangzhou, Zhejiang, China.