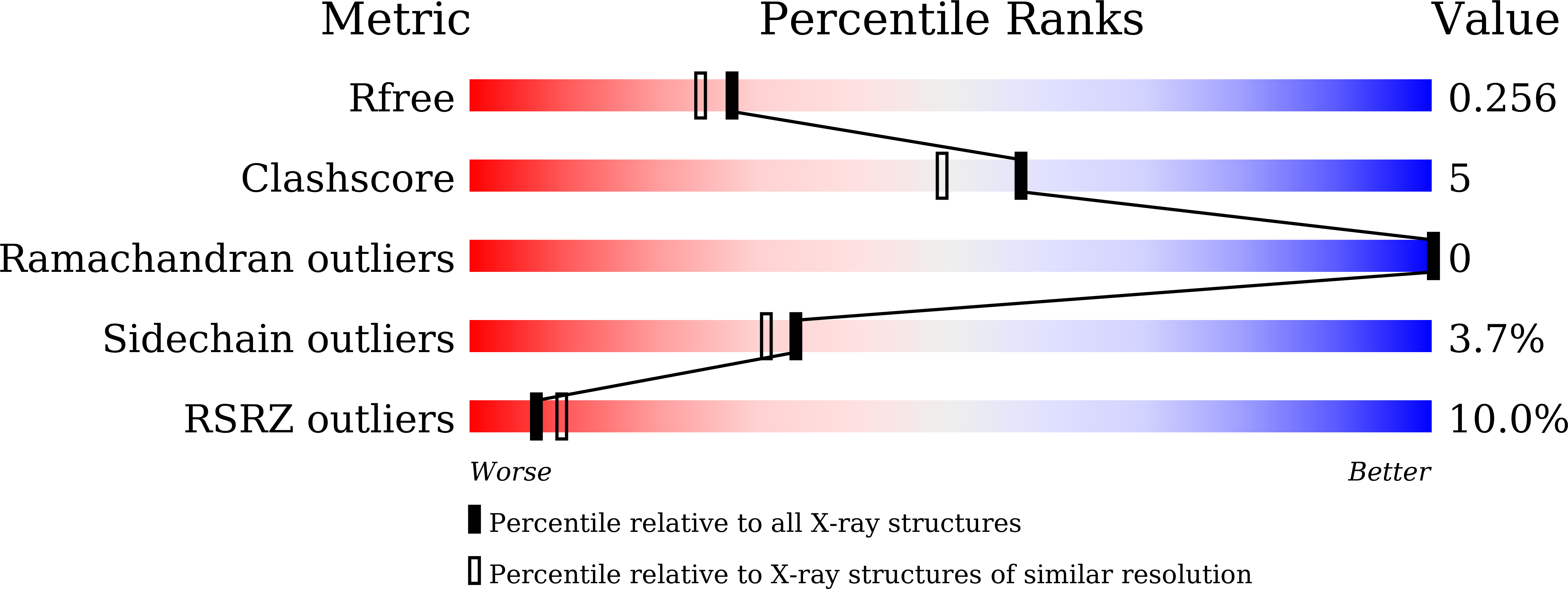
Structure-guided identification of novel dual-targeting estrogen receptor alpha degraders with aromatase inhibitory activity for the treatment of endocrine-resistant breast cancer.
Xin, L., Min, J., Hu, H., Li, Y., Du, C., Xie, B., Cheng, Y., Deng, X., Deng, X., Shen, K., Huang, J., Chen, C.C., Guo, R.T., Dong, C., Zhou, H.B.(2023) Eur J Med Chem 253: 115328-115328
- PubMed: 37037140
- DOI: https://doi.org/10.1016/j.ejmech.2023.115328
- Primary Citation of Related Structures:
7Y8F, 7Y8G - PubMed Abstract:
Drug resistance is a major challenge in conventional endocrine therapy for estrogen receptor (ER) positive breast cancer (BC). BC is a multifactorial disease, in which simultaneous aromatase (ARO) inhibition and ERα degradation may effectively inhibit the signal transduction of both proteins, thus potentially overcoming drug resistance caused by overexpression or mutation of target proteins. In this study, guided by the X-ray structure of a hit compound 30a in complex with ER-Y537S, a structure-based optimization was performed to get a series of multiacting inhibitors targeting both ERα and ARO, and finally a novel class of potent selective estrogen receptor degraders (SERDs) based on a three-dimensional oxabicycloheptene sulfonamide (OBHSA) scaffold equipped with aromatase inhibitor (AI) activity were identified. Of these dual-targeting SERD-AI hybrids, compound 31q incorporating a 1H-1,2,4-triazole moiety showed excellent ERα degradation activity, ARO inhibitory activity and remarkable antiproliferative activity against BC resistant cells. Furthermore, 31q manifested efficient tumor suppression in MCF-7 tumor xenograft models. Taken together, our study reported for the first time the highly efficient dual-targeting SERD-AI hybrid compounds, which may lay the foundation of translational research for improved treatment of endocrine-resistant BC.
Organizational Affiliation:
Department of Gynecological Oncology, Zhongnan Hospital of Wuhan University, School of Pharmaceutical Sciences, Wuhan University, Wuhan, 430071, China.