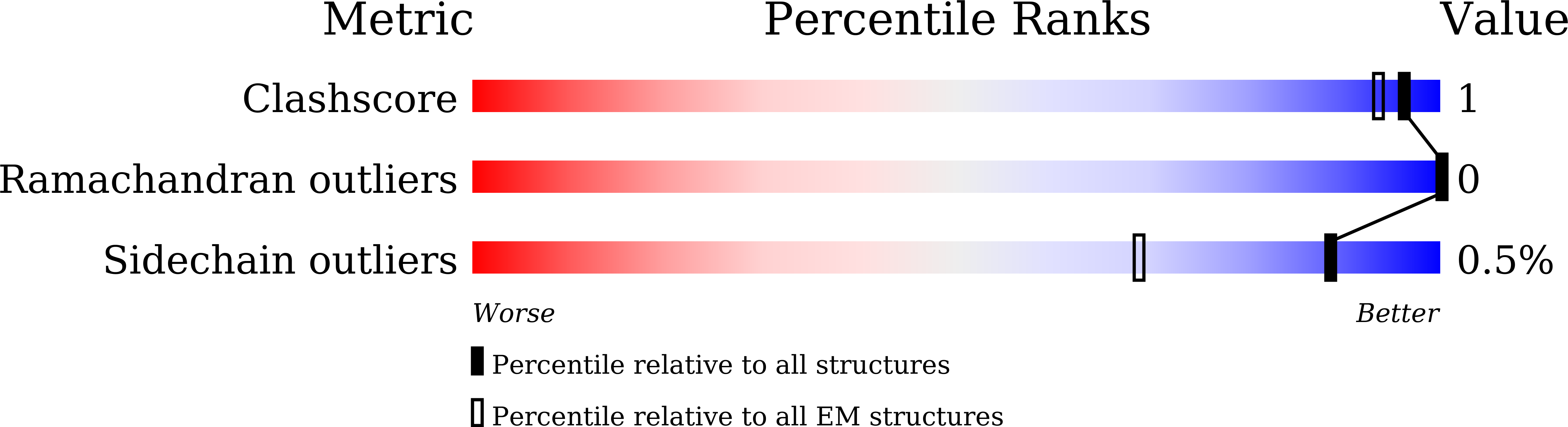Engineered disulfide reveals structural dynamics of locked SARS-CoV-2 spike.
Qu, K., Chen, Q., Ciazynska, K.A., Liu, B., Zhang, X., Wang, J., He, Y., Guan, J., He, J., Liu, T., Zhang, X., Carter, A.P., Xiong, X., Briggs, J.A.G.(2022) PLoS Pathog 18: e1010583-e1010583
- PubMed: 35905112
- DOI: https://doi.org/10.1371/journal.ppat.1010583
- Primary Citation of Related Structures:
7XTZ, 7XU0, 7XU1, 7XU2, 7XU3, 7XU4, 7XU5, 7XU6 - PubMed Abstract:
The spike (S) protein of SARS-CoV-2 has been observed in three distinct pre-fusion conformations: locked, closed and open. Of these, the function of the locked conformation remains poorly understood. Here we engineered a SARS-CoV-2 S protein construct "S-R/x3" to arrest SARS-CoV-2 spikes in the locked conformation by a disulfide bond. Using this construct we determined high-resolution structures confirming that the x3 disulfide bond has the ability to stabilize the otherwise transient locked conformations. Structural analyses reveal that wild-type SARS-CoV-2 spike can adopt two distinct locked-1 and locked-2 conformations. For the D614G spike, based on which all variants of concern were evolved, only the locked-2 conformation was observed. Analysis of the structures suggests that rigidified domain D in the locked conformations interacts with the hinge to domain C and thereby restrains RBD movement. Structural change in domain D correlates with spike conformational change. We propose that the locked-1 and locked-2 conformations of S are present in the acidic high-lipid cellular compartments during virus assembly and egress. In this model, release of the virion into the neutral pH extracellular space would favour transition to the closed or open conformations. The dynamics of this transition can be altered by mutations that modulate domain D structure, as is the case for the D614G mutation, leading to changes in viral fitness. The S-R/x3 construct provides a tool for the further structural and functional characterization of the locked conformations of S, as well as how sequence changes might alter S assembly and regulation of receptor binding domain dynamics.
Organizational Affiliation:
Structural Studies Division, Medical Research Council Laboratory of Molecular Biology, Cambridge, United Kingdom.