Characterization and Structural Determination of CmnG-A, the Adenylation Domain That Activates the Nonproteinogenic Amino Acid Capreomycidine in Capreomycin Biosynthesis.
Chen, I.H., Cheng, T., Wang, Y.L., Huang, S.J., Hsiao, Y.H., Lai, Y.T., Toh, S.I., Chu, J., Rudolf, J.D., Chang, C.Y.(2022) Chembiochem 23: e202200563-e202200563
- PubMed: 36278314
- DOI: https://doi.org/10.1002/cbic.202200563
- Primary Citation of Related Structures:
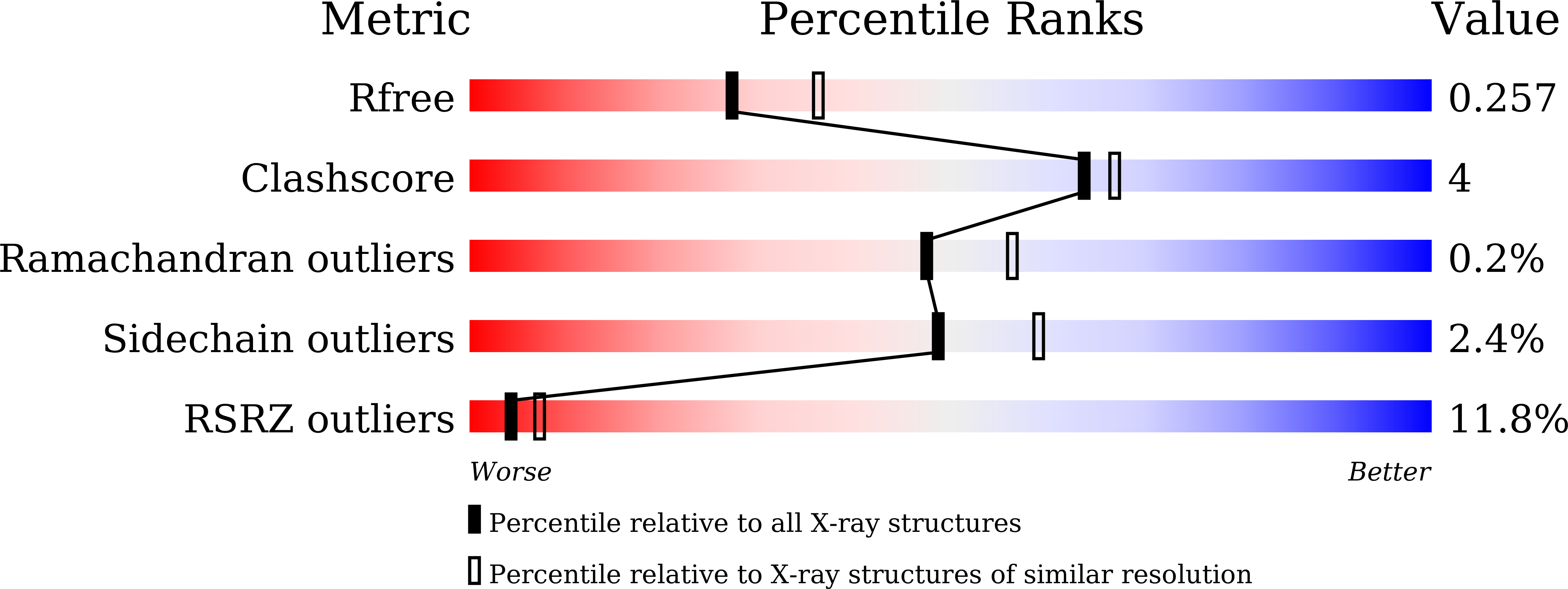
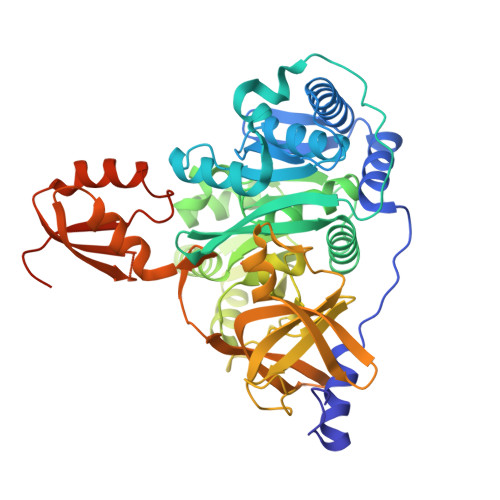
7XBS, 7XBT, 7XBU, 7XBV - PubMed Abstract:
Capreomycidine (Cap) is a nonproteinogenic amino acid and building block of nonribosomal peptide (NRP) natural products. We report the formation and activation of Cap in capreomycin biosynthesis. CmnC and CmnD catalyzed hydroxylation and cyclization, respectively, of l-Arg to form l-Cap. l-Cap is then adenylated by CmnG-A before being incorporated into the nonribosomal peptide. The co-crystal structures of CmnG-A with l-Cap and adenosine nucleotides provide insights into the specificity and engineering opportunities of this unique adenylation domain.
Organizational Affiliation:
Department of Biological Science and Technology, National Yang Ming Chiao Tung University, Hsinchu, 30010, Taiwan, ROC.