A specific G9a inhibitor unveils BGLT3 lncRNA as a universal mediator of chemically induced fetal globin gene expression.
Takase, S., Hiroyama, T., Shirai, F., Maemoto, Y., Nakata, A., Arata, M., Matsuoka, S., Sonoda, T., Niwa, H., Sato, S., Umehara, T., Shirouzu, M., Nishigaya, Y., Sumiya, T., Hashimoto, N., Namie, R., Usui, M., Ohishi, T., Ohba, S.I., Kawada, M., Hayashi, Y., Harada, H., Yamaguchi, T., Shinkai, Y., Nakamura, Y., Yoshida, M., Ito, A.(2023) Nat Commun 14: 23-23
- PubMed: 36635268
- DOI: https://doi.org/10.1038/s41467-022-35404-0
- Primary Citation of Related Structures:
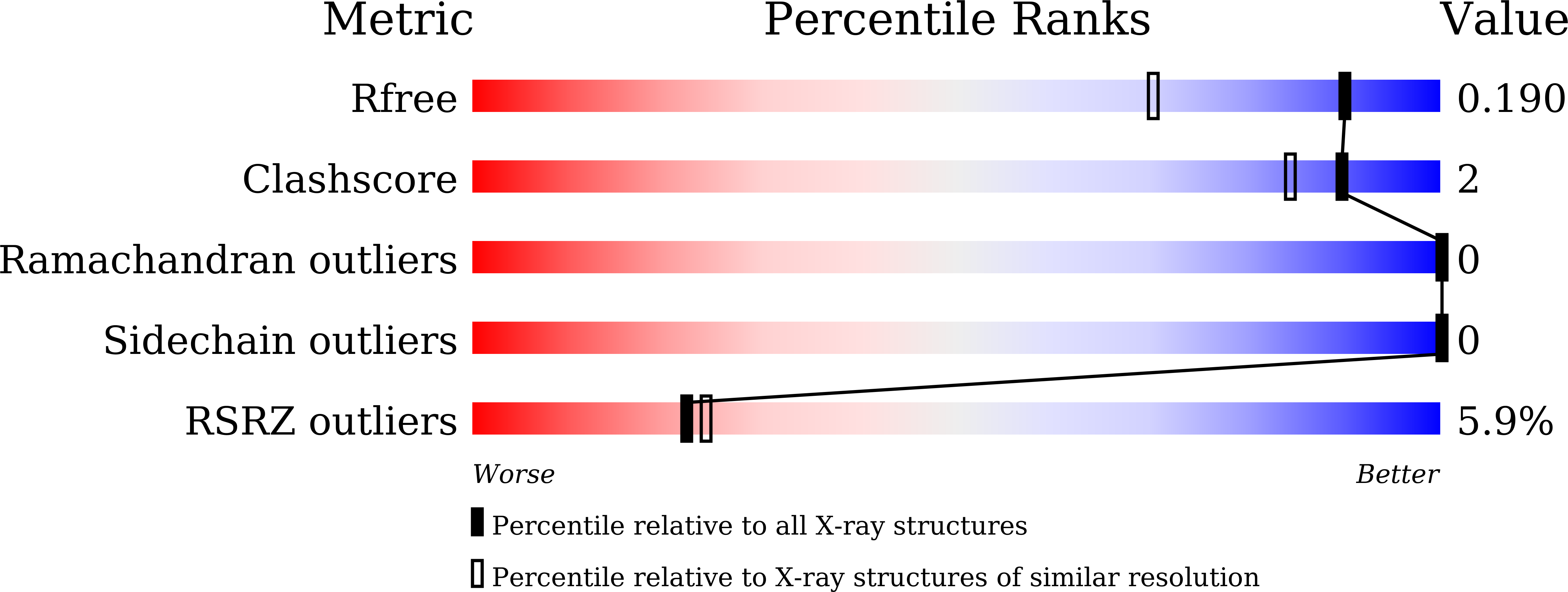
7X73 - PubMed Abstract:
Sickle cell disease (SCD) is a heritable disorder caused by β-globin gene mutations. Induction of fetal γ-globin is an established therapeutic strategy. Recently, epigenetic modulators, including G9a inhibitors, have been proposed as therapeutic agents. However, the molecular mechanisms whereby these small molecules reactivate γ-globin remain unclear. Here we report the development of a highly selective and non-genotoxic G9a inhibitor, RK-701. RK-701 treatment induces fetal globin expression both in human erythroid cells and in mice. Using RK-701, we find that BGLT3 long non-coding RNA plays an essential role in γ-globin induction. RK-701 selectively upregulates BGLT3 by inhibiting the recruitment of two major γ-globin repressors in complex with G9a onto the BGLT3 gene locus through CHD4, a component of the NuRD complex. Remarkably, BGLT3 is indispensable for γ-globin induction by not only RK-701 but also hydroxyurea and other inducers. The universal role of BGLT3 in γ-globin induction suggests its importance in SCD treatment.
Organizational Affiliation:
Laboratory of Cell Signaling, School of Life Sciences, Tokyo University of Pharmacy and Life Sciences, Hachioji, Tokyo, 192-0392, Japan.