Arylalkynyl amide-type peroxisome proliferator-activated receptor gamma (PPAR gamma )-selective antagonists covalently bind to the PPAR gamma ligand binding domain with a unique binding mode.
Yoshizawa, M., Aoyama, T., Itoh, T., Miyachi, H.(2022) Bioorg Med Chem Lett 64: 128676-128676
- PubMed: 35301139
- DOI: https://doi.org/10.1016/j.bmcl.2022.128676
- Primary Citation of Related Structures:
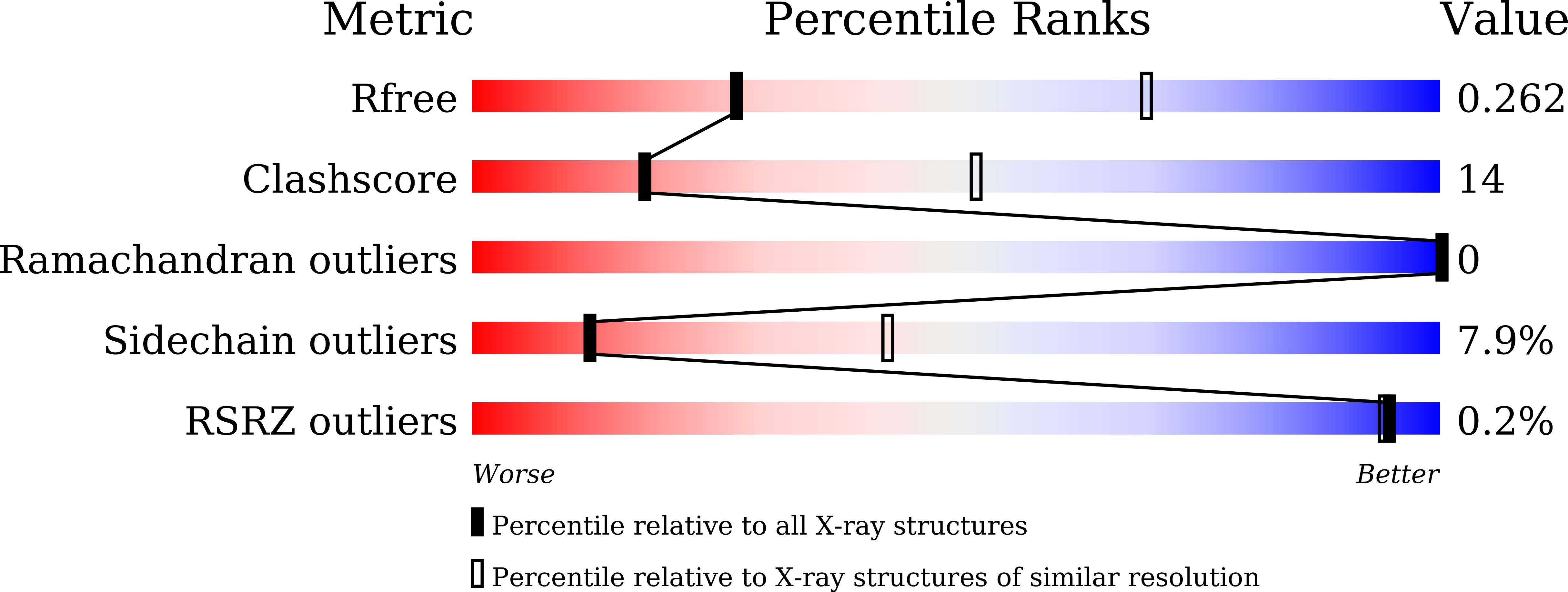
7WOX - PubMed Abstract:
Peroxisome proliferator-activated receptor γ (PPARγ) antagonists are drug candidates for the treatment of type 2 diabetes, obesity, and osteoporosis. Previously, we have designed and synthesized a series of substituted phenylalkynyl amide-type PPARγ antagonists. The representative compound, MMT-160, exhibited nanomolar-order PPARγ antagonistic activity. To understand the antagonistic mode of action of MMT-160, mass spectrometric and X-ray crystallographic analysis of MMT-160 in the presence of the PPARγ ligand binding domain (LBD) were performed. The mass spectrometry results clearly indicated that alkynyl amide-type PPARγ antagonists were covalently bound to the PPARγ LBD. The X-ray crystallographic analysis indicated that MMT-160 acted as a Michael acceptor and covalently bound to the PPARγ LBD via Cys285. In addition, MMT-160 bound to the PPARγ LBD with a binding mode that was different from the binding modes observed for PPARγ agonists and partial agonists.
Organizational Affiliation:
Laboratory of Drug Design and Medicinal Chemistry, Showa Pharmaceutical University, 3-3165 Higashi-Tamagawagakuen, Machida, Tokyo 194-8543, Japan.