Engineering Cytochrome P450BM3 Enzymes for Direct Nitration of Unsaturated Hydrocarbons.
Wang, X., Lin, X., Jiang, Y., Qin, X., Ma, N., Yao, F., Dong, S., Liu, C., Feng, Y., Jin, L., Xian, M., Cong, Z.(2023) Angew Chem Int Ed Engl 62: e202217678-e202217678
- PubMed: 36660956
- DOI: https://doi.org/10.1002/anie.202217678
- Primary Citation of Related Structures:
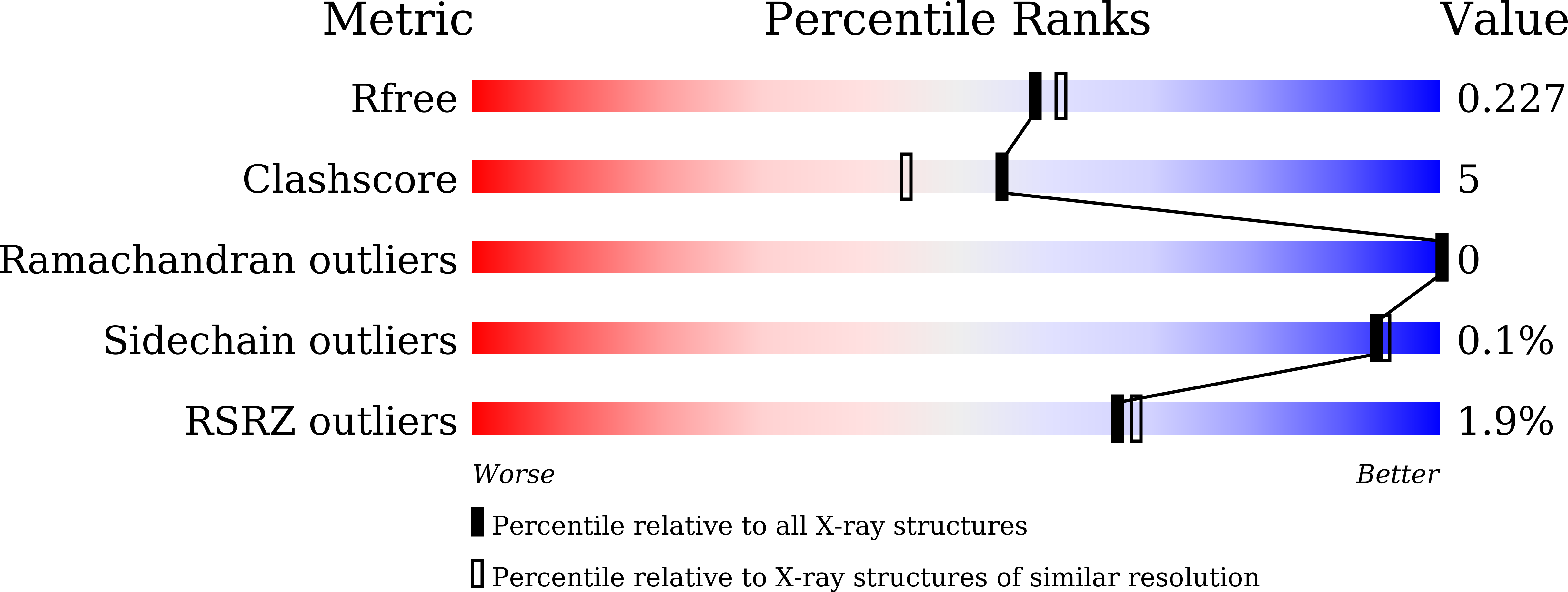
7WDG, 7WDH - PubMed Abstract:
Applications of the peroxidase activity of cytochrome P450 enzymes in synthetic chemistry remain largely unexplored. We present herein a protein engineering strategy to increase cytochrome P450BM3 peroxidase activity for the direct nitration of aromatic compounds and terminal aryl-substituted olefins in the presence of a dual-functional small molecule (DFSM). Site-directed mutations of key active-site residues allowed the efficient regulation of steric effects to limit substrate access and, thus, a significant decrease in monooxygenation activity and increase in peroxidase activity. Nitration of several phenol and aniline compounds also yielded ortho- and para-nitration products with moderate-to-high total turnover numbers. Besides direct aromatic nitration by P450 variants using nitrite as a nitrating agent, we also demonstrated the use of the DFSM-facilitated P450 peroxidase system for the nitration of the vinyl group of styrene and its derivatives.
Organizational Affiliation:
CAS Key Laboratory of Biofuels and Shandong Provincial Key Laboratory of Synthetic Biology, Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, Qingdao, Shandong, 266101, China.