The Structures of the Steroid Binding CYP142 Cytochrome P450 Enzymes from Mycobacterium ulcerans and Mycobacterium marinum.
Ghith, A., Doherty, D.Z., Bruning, J.B., Russell, R.A., De Voss, J.J., Bell, S.G.(2022) ACS Infect Dis 8: 1606-1617
- PubMed: 35881654
- DOI: https://doi.org/10.1021/acsinfecdis.2c00215
- Primary Citation of Related Structures:
7SH5, 7SMZ, 7TLO - PubMed Abstract:
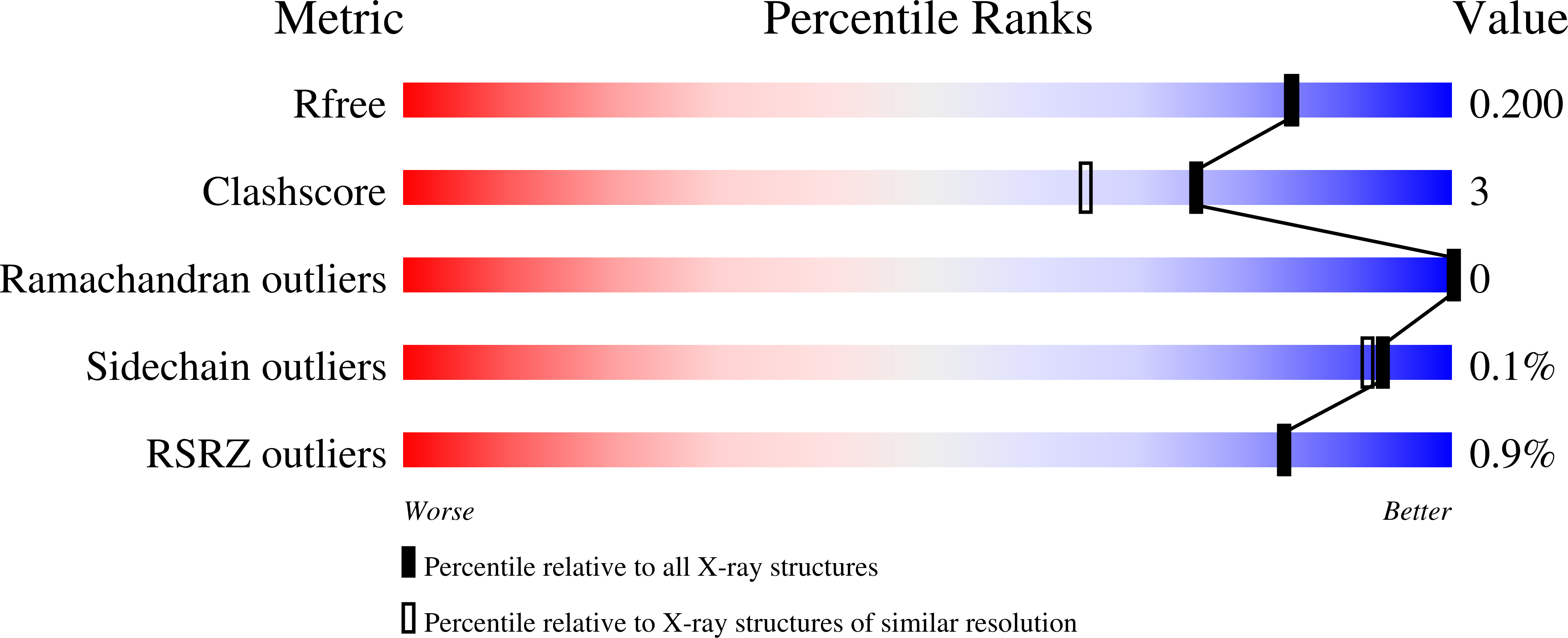
The steroid binding CYP142 cytochrome P450 enzymes of Mycobacterium species are involved in the metabolism of cholesterol and its derivatives. The equivalent enzyme from Mycobacterium ulcerans was studied to compare the degree of functional conservation between members of this CYP family. We compared substrate binding of the CYP142A3 enzymes of M. ulcerans and M. marinum and CYP142A1 from M. tuberculosis using UV-vis spectroscopy. The catalytic oxidation of cholesterol derivatives by all three enzymes was undertaken. Both CYP142A3 enzymes were structurally characterized by X-ray crystallography. The amino acid sequences of the CYP142A3 enzymes are more similar to CYP142A1 from M. tuberculosis than CYP142A2 from Mycolicibacterium smegmatis . Both CYP142A3 enzymes have substrate binding properties, which are more resemblant to CYP142A1 than CYP142A2. The cholest-4-en-3-one-bound X-ray crystal structure of both CYP142A3 enzymes were determined at a resolution of <1.8 Å, revealing the substrate binding mode at a high level of detail. The structures of the cholest-4-en-3-one binding CYP142 enzymes from M. ulcerans and M. marinum demonstrate how the steroid binds in the active site of these enzymes. They provide an explanation for the high selectivity of the enzyme for terminal methyl C-H bond oxidation to form 26-hydroxy derivatives. These enzymes in pathogenic Mycobacterium species are candidates for inhibition. The work here demonstrates that similar drug molecules could target these CYP142 enzymes from different species in order to combat Buruli ulcer or tuberculosis.
Organizational Affiliation:
Department of Chemistry, University of Adelaide, Adelaide, SA 5005, Australia.