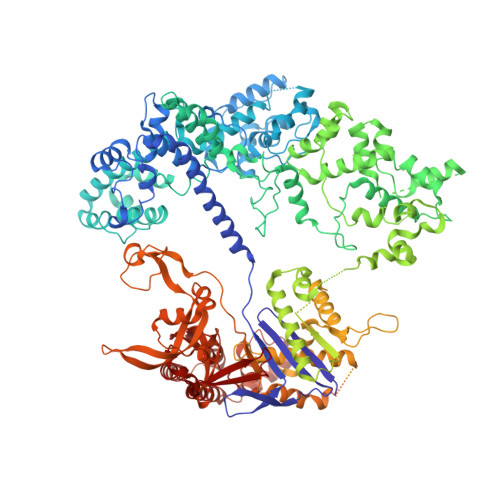
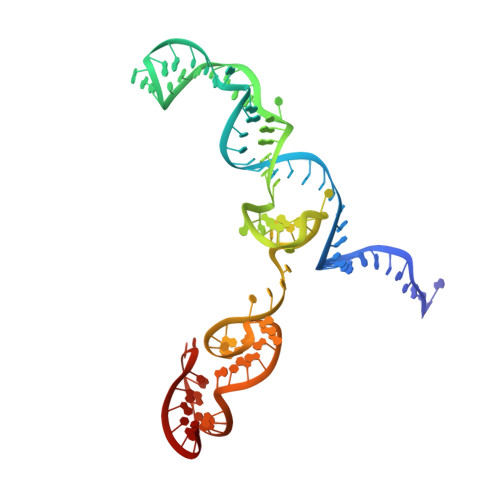
Structural basis for mismatch surveillance by CRISPR-Cas9.
Bravo, J.P.K., Liu, M.S., Hibshman, G.N., Dangerfield, T.L., Jung, K., McCool, R.S., Johnson, K.A., Taylor, D.W.(2022) Nature 603: 343-347
- PubMed: 35236982
- DOI: https://doi.org/10.1038/s41586-022-04470-1
- Primary Citation of Related Structures:
7S4U, 7S4V, 7S4X - PubMed Abstract:
CRISPR-Cas9 as a programmable genome editing tool is hindered by off-target DNA cleavage 1-4 , and the underlying mechanisms by which Cas9 recognizes mismatches are poorly understood 5-7 . Although Cas9 variants with greater discrimination against mismatches have been designed 8-10 , these suffer from substantially reduced rates of on-target DNA cleavage 5,11 . Here we used kinetics-guided cryo-electron microscopy to determine the structure of Cas9 at different stages of mismatch cleavage. We observed a distinct, linear conformation of the guide RNA-DNA duplex formed in the presence of mismatches, which prevents Cas9 activation. Although the canonical kinked guide RNA-DNA duplex conformation facilitates DNA cleavage, we observe that substrates that contain mismatches distal to the protospacer adjacent motif are stabilized by reorganization of a loop in the RuvC domain. Mutagenesis of mismatch-stabilizing residues reduces off-target DNA cleavage but maintains rapid on-target DNA cleavage. By targeting regions that are exclusively involved in mismatch tolerance, we provide a proof of concept for the design of next-generation high-fidelity Cas9 variants.
Organizational Affiliation:
Department of Molecular Biosciences, University of Texas at Austin, Austin, TX, USA.