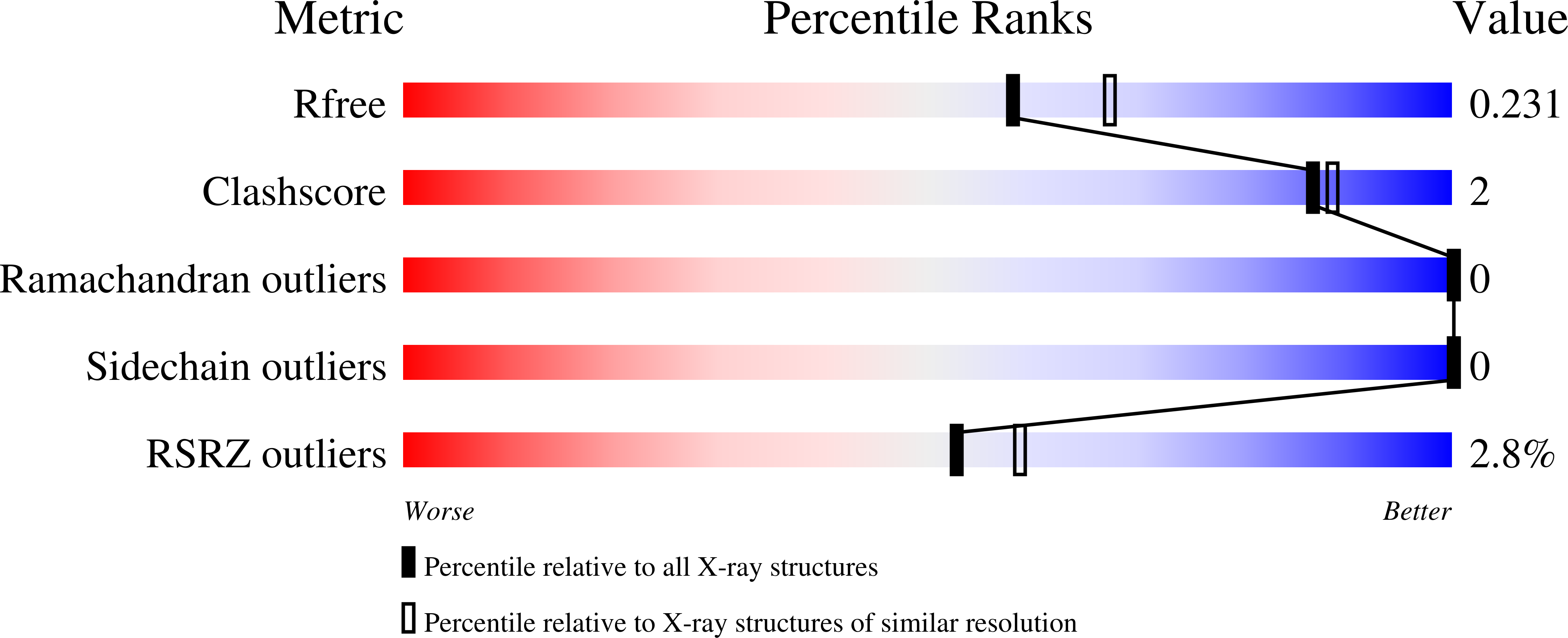
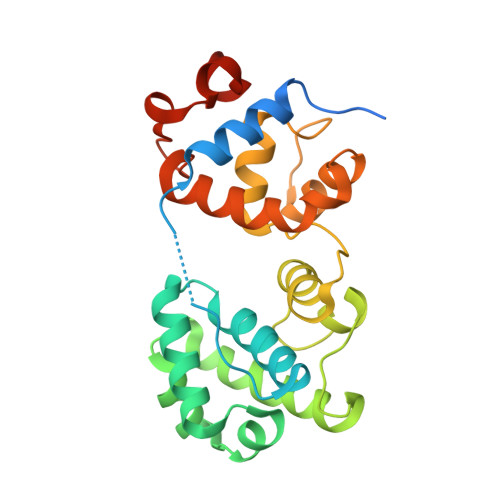
Caught in motion: human NTHL1 undergoes interdomain rearrangement necessary for catalysis.
Carroll, B.L., Zahn, K.E., Hanley, J.P., Wallace, S.S., Dragon, J.A., Doublie, S.(2021) Nucleic Acids Res 49: 13165-13178
- PubMed: 34871433
- DOI: https://doi.org/10.1093/nar/gkab1162
- Primary Citation of Related Structures:
7RDS, 7RDT - PubMed Abstract:
Base excision repair (BER) is the main pathway protecting cells from the continuous damage to DNA inflicted by reactive oxygen species. BER is initiated by DNA glycosylases, each of which repairs a particular class of base damage. NTHL1, a bifunctional DNA glycosylase, possesses both glycolytic and β-lytic activities with a preference for oxidized pyrimidine substrates. Defects in human NTHL1 drive a class of polyposis colorectal cancer. We report the first X-ray crystal structure of hNTHL1, revealing an open conformation not previously observed in the bacterial orthologs. In this conformation, the six-helical barrel domain comprising the helix-hairpin-helix (HhH) DNA binding motif is tipped away from the iron sulphur cluster-containing domain, requiring a conformational change to assemble a catalytic site upon DNA binding. We found that the flexibility of hNTHL1 and its ability to adopt an open configuration can be attributed to an interdomain linker. Swapping the human linker sequence for that of Escherichia coli yielded a protein chimera that crystallized in a closed conformation and had a reduced activity on lesion-containing DNA. This large scale interdomain rearrangement during catalysis is unprecedented for a HhH superfamily DNA glycosylase and provides important insight into the molecular mechanism of hNTHL1.
Organizational Affiliation:
Department of Microbiology and Molecular Genetics, University of Vermont, Burlington, VT 05405, USA.