One-Step Synthesis of Photoaffinity Probes for Live-Cell MS-Based Proteomics.
Fallon, D.J., Lehmann, S., Chung, C.W., Phillipou, A., Eberl, C., Fantom, K.G.M., Zappacosta, F., Patel, V.K., Bantscheff, M., Schofield, C.J., Tomkinson, N.C.O., Bush, J.T.(2021) Chemistry 27: 17880-17888
- PubMed: 34328642
- DOI: https://doi.org/10.1002/chem.202102036
- Primary Citation of Related Structures:
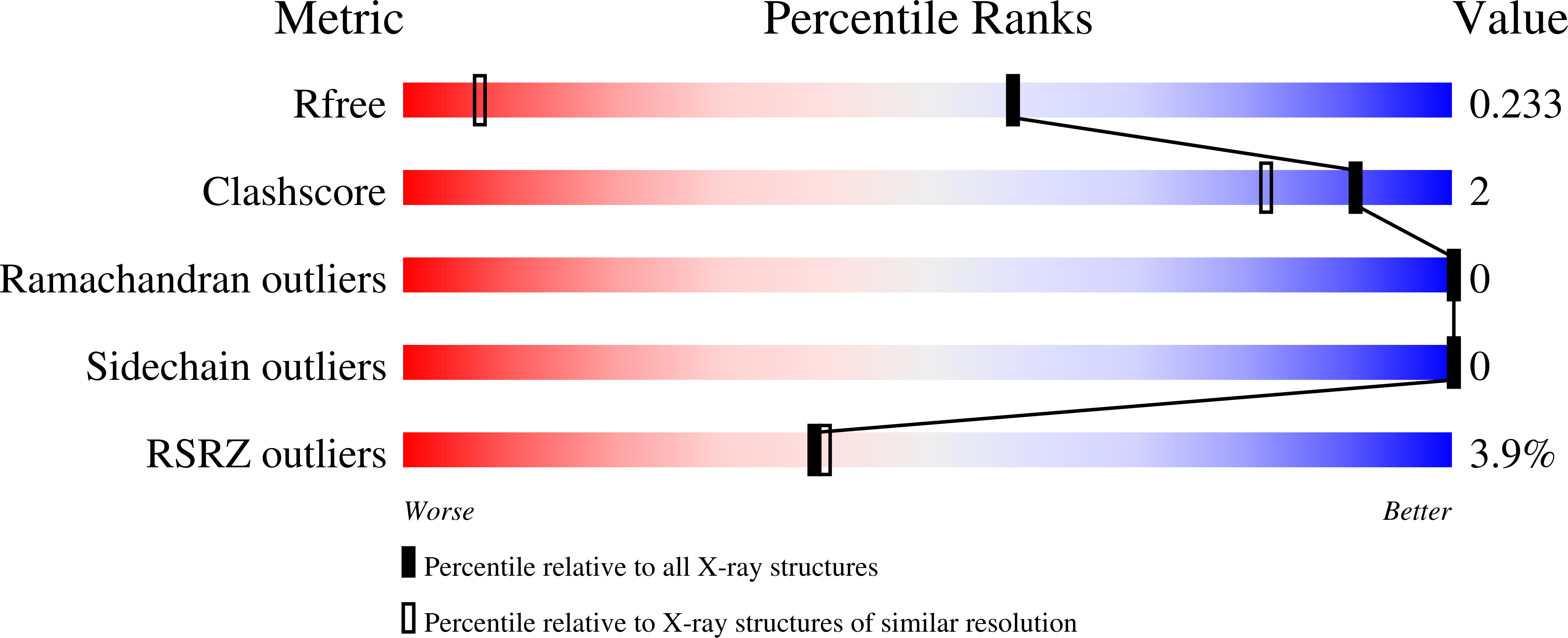
7P6V, 7P6W, 7P6Y - PubMed Abstract:
We present a one-step Ugi reaction protocol for the expedient synthesis of photoaffinity probes for live-cell MS-based proteomics. The reaction couples an amine affinity function with commonly used photoreactive groups, and a variety of handle functionalities. Using this technology, a series of pan-BET (BET: bromodomain and extra-terminal domain) selective bromodomain photoaffinity probes were obtained by parallel synthesis. Studies on the effects of photoreactive group, linker length and irradiation wavelength on photocrosslinking efficiency provide valuable insights into photoaffinity probe design. Optimal probes were progressed to MS-based proteomics to capture the BET family of proteins from live cells and reveal their potential on- and off-target profiles.
Organizational Affiliation:
GlaxoSmithKline R&D, Gunnels Wood Road, Stevenage, SG1 2NY, UK.