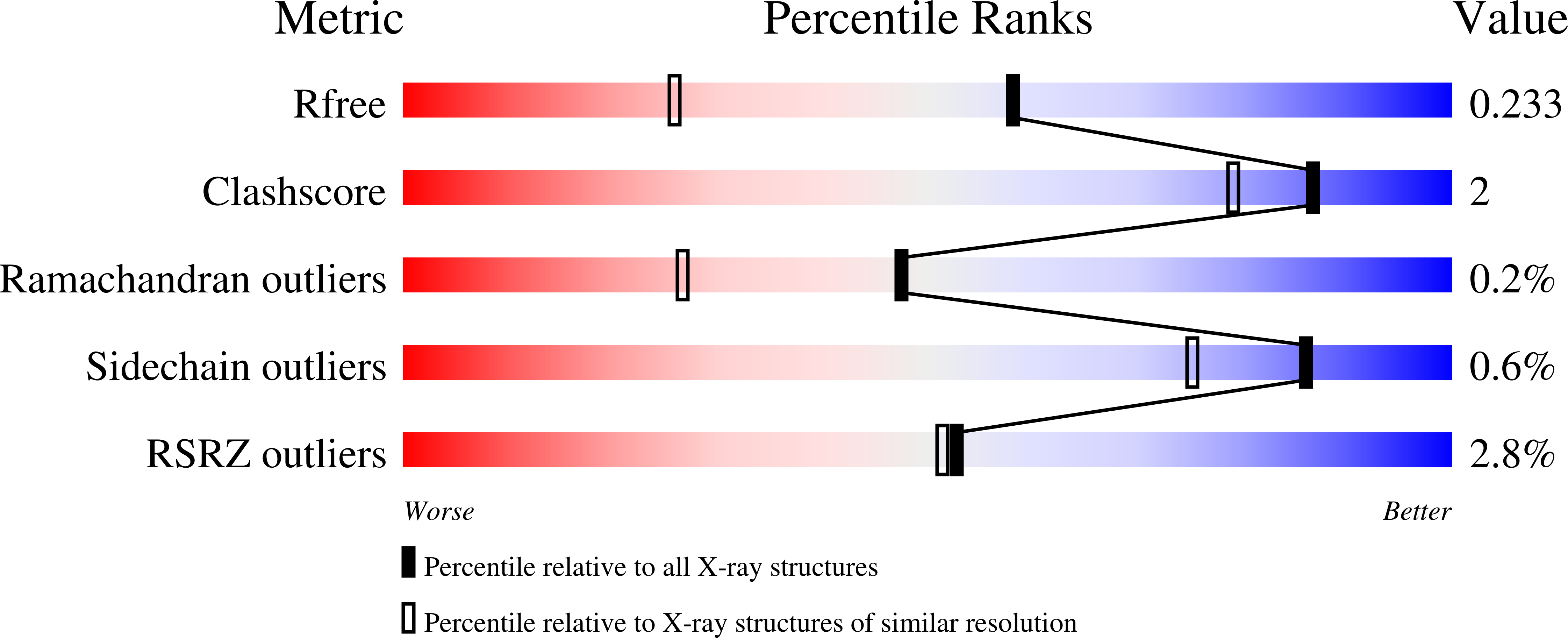
Debottlenecking 4-hydroxybenzoate hydroxylation in Pseudomonas putida KT2440 improves muconate productivity from p-coumarate.
Kuatsjah, E., Johnson, C.W., Salvachua, D., Werner, A.Z., Zahn, M., Szostkiewicz, C.J., Singer, C.A., Dominick, G., Okekeogbu, I., Haugen, S.J., Woodworth, S.P., Ramirez, K.J., Giannone, R.J., Hettich, R.L., McGeehan, J.E., Beckham, G.T.(2022) Metab Eng 70: 31-42
- PubMed: 34982998
- DOI: https://doi.org/10.1016/j.ymben.2021.12.010
- Primary Citation of Related Structures:
7ON9 - PubMed Abstract:
The transformation of 4-hydroxybenzoate (4-HBA) to protocatechuate (PCA) is catalyzed by flavoprotein oxygenases known as para-hydroxybenzoate-3-hydroxylases (PHBHs). In Pseudomonas putida KT2440 (P. putida) strains engineered to convert lignin-related aromatic compounds to muconic acid (MA), PHBH activity is rate-limiting, as indicated by the accumulation of 4-HBA, which ultimately limits MA productivity. Here, we hypothesized that replacement of PobA, the native P. putida PHBH, with PraI, a PHBH from Paenibacillus sp. JJ-1b with a broader nicotinamide cofactor preference, could alleviate this bottleneck. Biochemical assays confirmed the strict preference of NADPH for PobA, while PraI can utilize either NADH or NADPH. Kinetic assays demonstrated that both PobA and PraI can utilize NADPH with comparable catalytic efficiency and that PraI also efficiently utilizes NADH at roughly half the catalytic efficiency. The X-ray crystal structure of PraI was solved and revealed absolute conservation of the active site architecture to other PHBH structures despite their differing cofactor preferences. To understand the effect in vivo, we compared three P. putida strains engineered to produce MA from p-coumarate (pCA), showing that expression of praI leads to lower 4-HBA accumulation and decreased NADP + /NADPH ratios relative to strains harboring pobA, indicative of a relieved 4-HBA bottleneck due to increased NADPH availability. In bioreactor cultivations, a strain exclusively expressing praI achieved a titer of 40 g/L MA at 100% molar yield and a productivity of 0.5 g/L/h. Overall, this study demonstrates the benefit of sampling readily available natural enzyme diversity for debottlenecking metabolic flux in an engineered strain for microbial conversion of lignin-derived compounds to value-added products.
Organizational Affiliation:
Renewable Resources and Enabling Sciences Center, National Renewable Energy Laboratory, Golden, CO, 80401, USA; Center for Bioenergy Innovation, Oak Ridge National Laboratory, Oak Ridge, TN, 37830, USA.