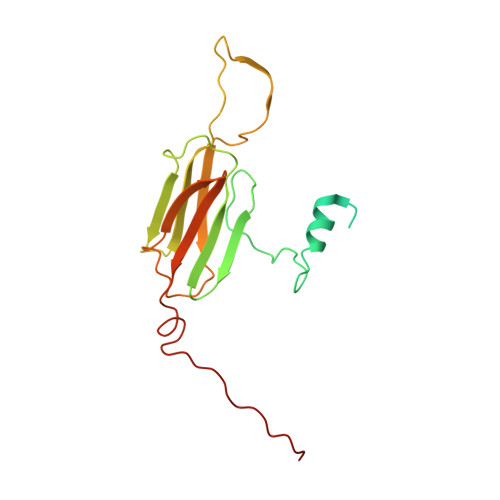
Phosphorylation activates the yeast small heat shock protein Hsp26 by weakening domain contacts in the oligomer ensemble.
Muhlhofer, M., Peters, C., Kriehuber, T., Kreuzeder, M., Kazman, P., Rodina, N., Reif, B., Haslbeck, M., Weinkauf, S., Buchner, J.(2021) Nat Commun 12: 6697-6697
- PubMed: 34795272
- DOI: https://doi.org/10.1038/s41467-021-27036-7
- Primary Citation of Related Structures:
7OA6 - PubMed Abstract:
Hsp26 is a small heat shock protein (sHsp) from S. cerevisiae. Its chaperone activity is activated by oligomer dissociation at heat shock temperatures. Hsp26 contains 9 phosphorylation sites in different structural elements. Our analysis of phospho-mimetic mutations shows that phosphorylation activates Hsp26 at permissive temperatures. The cryo-EM structure of the Hsp26 40mer revealed contacts between the conserved core domain of Hsp26 and the so-called thermosensor domain in the N-terminal part of the protein, which are targeted by phosphorylation. Furthermore, several phosphorylation sites in the C-terminal extension, which link subunits within the oligomer, are sensitive to the introduction of negative charges. In all cases, the intrinsic inhibition of chaperone activity is relieved and the N-terminal domain becomes accessible for substrate protein binding. The weakening of domain interactions within and between subunits by phosphorylation to activate the chaperone activity in response to proteotoxic stresses independent of heat stress could be a general regulation principle of sHsps.
Organizational Affiliation:
Center for Protein Assemblies, Department of Chemistry, Technische Universität München, Ernst-Otto-Fischer Str. 8, 85747, Garching, Germany.