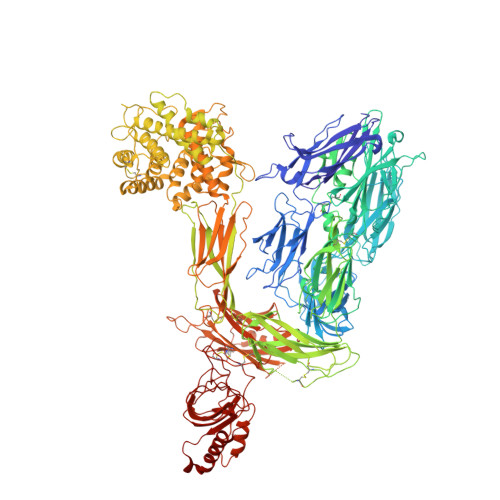
Structural basis of soluble membrane attack complex packaging for clearance.
Menny, A., Lukassen, M.V., Couves, E.C., Franc, V., Heck, A.J.R., Bubeck, D.(2021) Nat Commun 12: 6086-6086
- PubMed: 34667172
- DOI: https://doi.org/10.1038/s41467-021-26366-w
- Primary Citation of Related Structures:
7NYC, 7NYD - PubMed Abstract:
Unregulated complement activation causes inflammatory and immunological pathologies with consequences for human disease. To prevent bystander damage during an immune response, extracellular chaperones (clusterin and vitronectin) capture and clear soluble precursors to the membrane attack complex (sMAC). However, how these chaperones block further polymerization of MAC and prevent the complex from binding target membranes remains unclear. Here, we address that question by combining cryo electron microscopy (cryoEM) and cross-linking mass spectrometry (XL-MS) to solve the structure of sMAC. Together our data reveal how clusterin recognizes and inhibits polymerizing complement proteins by binding a negatively charged surface of sMAC. Furthermore, we show that the pore-forming C9 protein is trapped in an intermediate conformation whereby only one of its two transmembrane β-hairpins has unfurled. This structure provides molecular details for immune pore formation and helps explain a complement control mechanism that has potential implications for how cell clearance pathways mediate immune homeostasis.
Organizational Affiliation:
Department of Life Sciences, Sir Ernst Chain Building, Imperial College London, London, SW7 2AZ, UK.