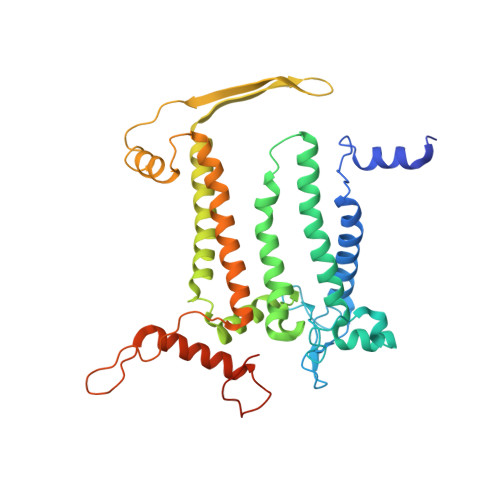
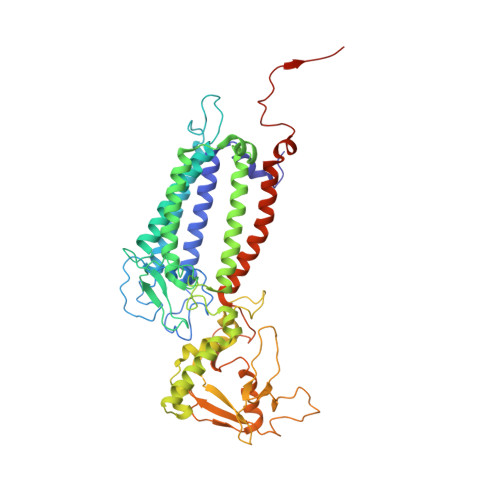
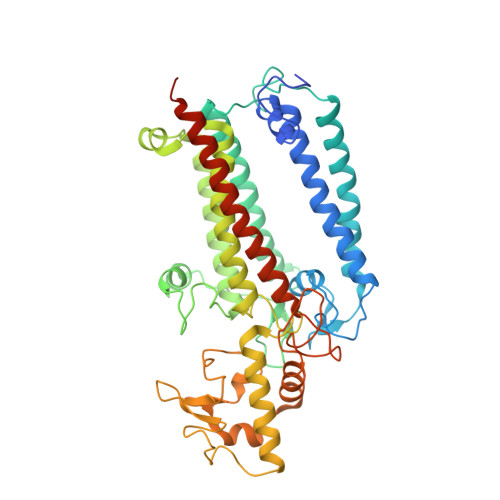
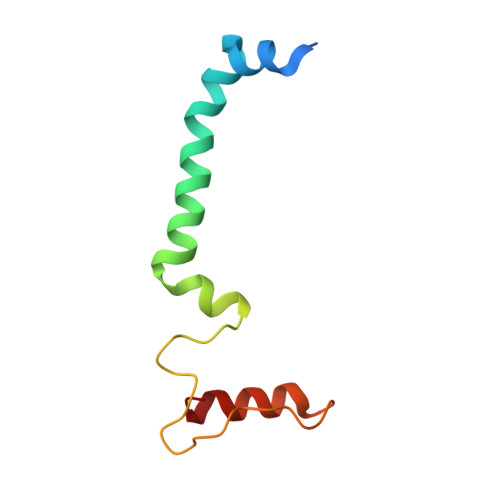
Structural insights into photosystem II assembly.
Zabret, J., Bohn, S., Schuller, S.K., Arnolds, O., Moller, M., Meier-Credo, J., Liauw, P., Chan, A., Tajkhorshid, E., Langer, J.D., Stoll, R., Krieger-Liszkay, A., Engel, B.D., Rudack, T., Schuller, J.M., Nowaczyk, M.M.(2021) Nat Plants 7: 524-538
- PubMed: 33846594
- DOI: https://doi.org/10.1038/s41477-021-00895-0
- Primary Citation of Related Structures:
7NHO, 7NHP, 7NHQ - PubMed Abstract:
Biogenesis of photosystem II (PSII), nature's water-splitting catalyst, is assisted by auxiliary proteins that form transient complexes with PSII components to facilitate stepwise assembly events. Using cryo-electron microscopy, we solved the structure of such a PSII assembly intermediate from Thermosynechococcus elongatus at 2.94 Å resolution. It contains three assembly factors (Psb27, Psb28 and Psb34) and provides detailed insights into their molecular function. Binding of Psb28 induces large conformational changes at the PSII acceptor side, which distort the binding pocket of the mobile quinone (Q B ) and replace the bicarbonate ligand of non-haem iron with glutamate, a structural motif found in reaction centres of non-oxygenic photosynthetic bacteria. These results reveal mechanisms that protect PSII from damage during biogenesis until water splitting is activated. Our structure further demonstrates how the PSII active site is prepared for the incorporation of the Mn 4 CaO 5 cluster, which performs the unique water-splitting reaction.
Organizational Affiliation:
Department of Plant Biochemistry, Faculty of Biology and Biotechnology, Ruhr University Bochum, Bochum, Germany.