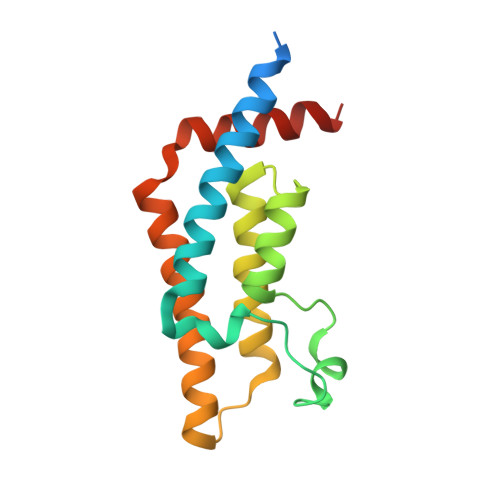
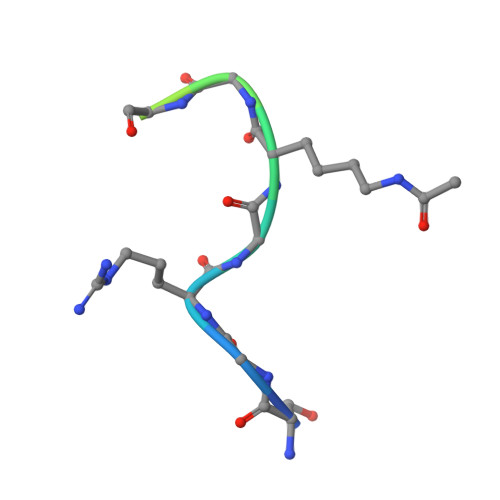
Coordination of Di-Acetylated Histone Ligands by the ATAD2 Bromodomain.
Evans, C.M., Phillips, M., Malone, K.L., Tonelli, M., Cornilescu, G., Cornilescu, C., Holton, S.J., Gorjanacz, M., Wang, L., Carlson, S., Gay, J.C., Nix, J.C., Demeler, B., Markley, J.L., Glass, K.C.(2021) Int J Mol Sci 22
- PubMed: 34502039
- DOI: https://doi.org/10.3390/ijms22179128
- Primary Citation of Related Structures:
7M98 - PubMed Abstract:
The ATPase Family, AAA domain-containing protein 2 (ATAD2) bromodomain (BRD) has a canonical bromodomain structure consisting of four α-helices. ATAD2 functions as a co-activator of the androgen and estrogen receptors as well as the MYC and E2F transcription factors. ATAD2 also functions during DNA replication, recognizing newly synthesized histones. In addition, ATAD2 is shown to be up-regulated in multiple forms of cancer including breast, lung, gastric, endometrial, renal, and prostate. Furthermore, up-regulation of ATAD2 is strongly correlated with poor prognosis in many types of cancer, making the ATAD2 bromodomain an innovative target for cancer therapeutics. In this study, we describe the recognition of histone acetyllysine modifications by the ATAD2 bromodomain. Residue-specific information on the complex formed between the histone tail and the ATAD2 bromodomain, obtained through nuclear magnetic resonance spectroscopy (NMR) and X-ray crystallography, illustrates key residues lining the binding pocket, which are involved in coordination of di-acetylated histone tails. Analytical ultracentrifugation, NMR relaxation data, and isothermal titration calorimetry further confirm the monomeric state of the functionally active ATAD2 bromodomain in complex with di-acetylated histone ligands. Overall, we describe histone tail recognition by ATAD2 BRD and illustrate that one acetyllysine group is primarily engaged by the conserved asparagine (N1064), the "RVF" shelf residues, and the flexible ZA loop. Coordination of a second acetyllysine group also occurs within the same binding pocket but is essentially governed by unique hydrophobic and electrostatic interactions making the di-acetyllysine histone coordination more specific than previously presumed.
Organizational Affiliation:
Department of Pharmaceutical Sciences, Albany College of Pharmacy and Health Sciences, Colchester, VT 05446, USA.