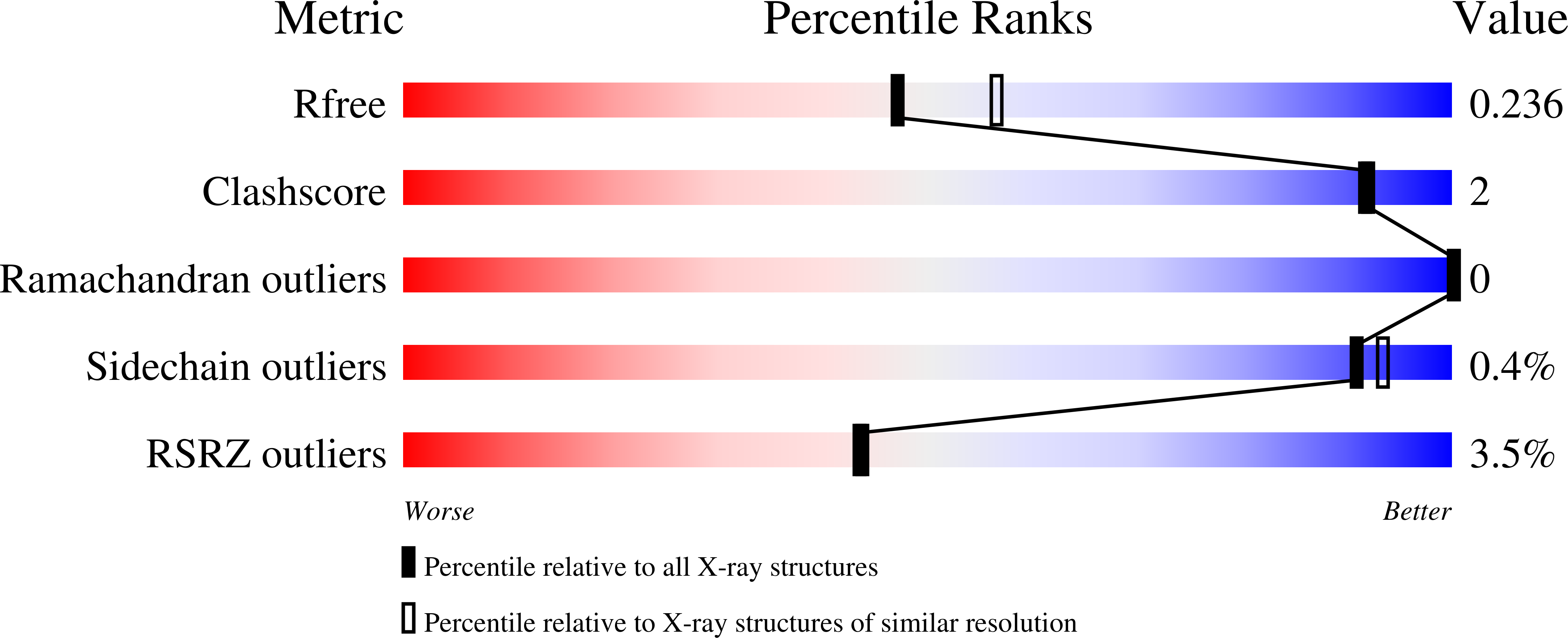
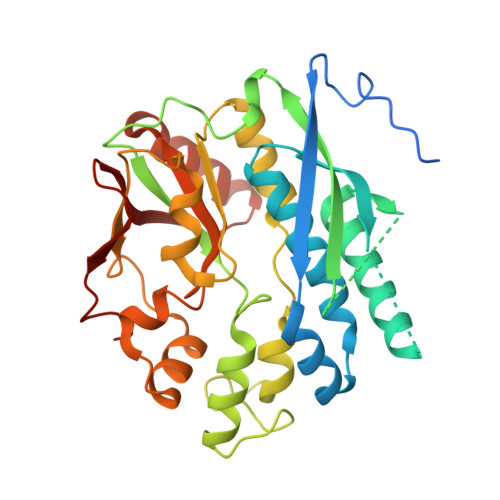
Structural features of Cryptococcus neoformans bifunctional GAR/AIR synthetase may present novel antifungal drug targets.
Chua, S.M.H., Wizrah, M.S.I., Luo, Z., Lim, B.Y.J., Kappler, U., Kobe, B., Fraser, J.A.(2021) J Biol Chem 297: 101091-101091
- PubMed: 34416230
- DOI: https://doi.org/10.1016/j.jbc.2021.101091
- Primary Citation of Related Structures:
7LVO, 7LVP - PubMed Abstract:
Cryptococcus neoformans is a fungus that causes life-threatening systemic mycoses. During infection of the human host, this pathogen experiences a major change in the availability of purines; the fungus can scavenge the abundant purines in its environmental niche of pigeon excrement, but must employ de novo biosynthesis in the purine-poor human CNS. Eleven sequential enzymatic steps are required to form the first purine base, IMP, an intermediate in the formation of ATP and GTP. Over the course of evolution, several gene fusion events led to the formation of multifunctional purine biosynthetic enzymes in most organisms, particularly the higher eukaryotes. In C. neoformans, phosphoribosyl-glycinamide synthetase (GARs) and phosphoribosyl-aminoimidazole synthetase (AIRs) are fused into a bifunctional enzyme, while the human ortholog is a trifunctional enzyme that also includes GAR transformylase. Here we functionally, biochemically, and structurally characterized C. neoformans GARs and AIRs to identify drug targetable features. GARs/AIRs are essential for de novo purine production and virulence in a murine inhalation infection model. Characterization of GARs enzymatic functional parameters showed that C. neoformans GARs/AIRs have lower affinity for substrates glycine and PRA compared with the trifunctional metazoan enzyme. The crystal structure of C. neoformans GARs revealed differences in the glycine- and ATP-binding sites compared with the Homo sapiens enzyme, while the crystal structure of AIRs shows high structural similarity compared with its H. sapiens ortholog as a monomer but differences as a dimer. The alterations in functional and structural characteristics between fungal and human enzymes could potentially be exploited for antifungal development.
Organizational Affiliation:
Australian Infectious Diseases Research Centre, The University of Queensland, St Lucia, Queensland, Australia; School of Chemistry & Molecular Biosciences, The University of Queensland, St Lucia, Queensland, Australia.