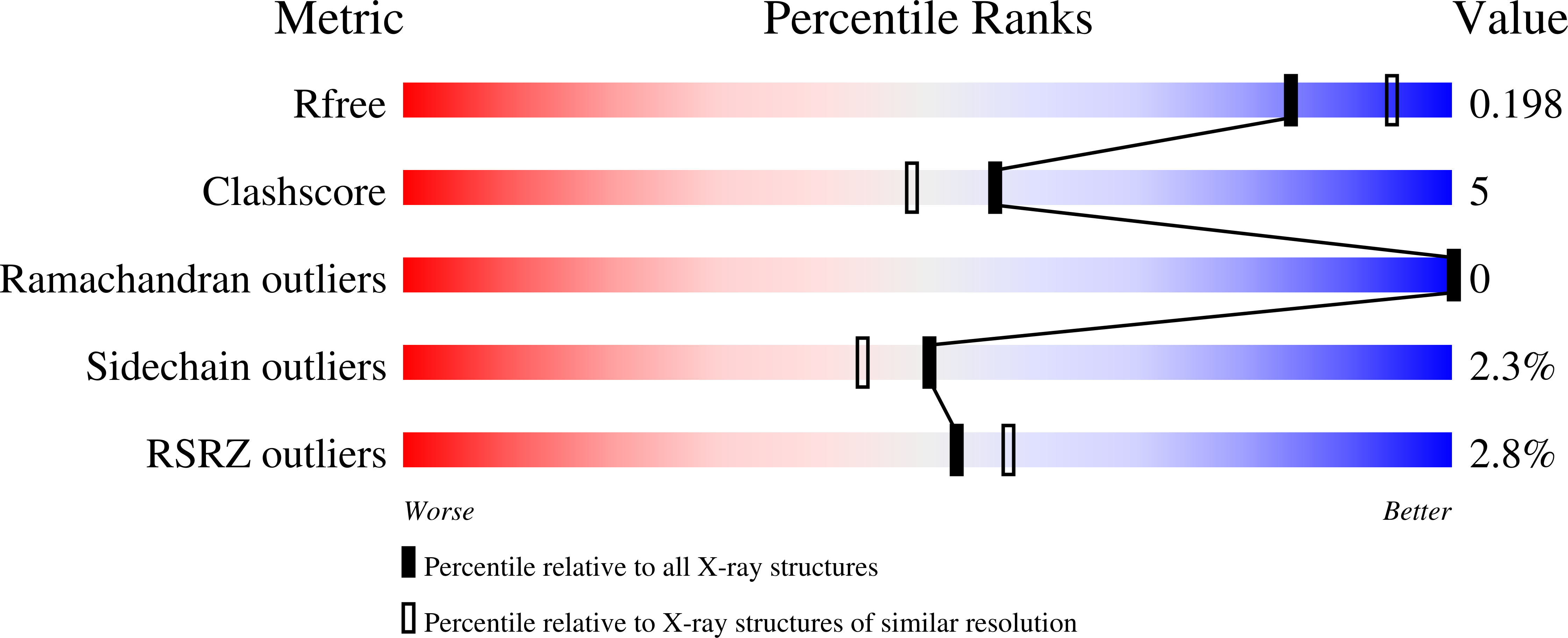
Structural and molecular rationale for the diversification of resistance mediated by the Antibiotic_NAT family.
Stogios, P.J., Bordeleau, E., Xu, Z., Skarina, T., Evdokimova, E., Chou, S., Diorio-Toth, L., D'Souza, A.W., Patel, S., Dantas, G., Wright, G.D., Savchenko, A.(2022) Commun Biol 5: 263-263
- PubMed: 35338238
- DOI: https://doi.org/10.1038/s42003-022-03219-w
- Primary Citation of Related Structures:
5HT0, 6MMZ, 6MN0, 6MN3, 6MN4, 6MN5, 7KES, 7LAO, 7LAP - PubMed Abstract:
The environmental microbiome harbors a vast repertoire of antibiotic resistance genes (ARGs) which can serve as evolutionary predecessors for ARGs found in pathogenic bacteria, or can be directly mobilized to pathogens in the presence of selection pressures. Thus, ARGs from benign environmental bacteria are an important resource for understanding clinically relevant resistance. Here, we conduct a comprehensive functional analysis of the Antibiotic_NAT family of aminoglycoside acetyltransferases. We determined a pan-family antibiogram of 21 Antibiotic_NAT enzymes, including 8 derived from clinical isolates and 13 from environmental metagenomic samples. We find that environment-derived representatives confer high-level, broad-spectrum resistance, including against the atypical aminoglycoside apramycin, and that a metagenome-derived gene likely is ancestral to an aac(3) gene found in clinical isolates. Through crystallographic analysis, we rationalize the molecular basis for diversification of substrate specificity across the family. This work provides critical data on the molecular mechanism underpinning resistance to established and emergent aminoglycoside antibiotics and broadens our understanding of ARGs in the environment.
Organizational Affiliation:
Department of Chemical Engineering and Applied Chemistry, University of Toronto, Toronto, M5S 3E5, Canada.