A chemical genetics approach to examine the functions of AAA proteins.
Cupido, T., Jones, N.H., Grasso, M.J., Pisa, R., Kapoor, T.M.(2021) Nat Struct Mol Biol 28: 388-397
- PubMed: 33782614
- DOI: https://doi.org/10.1038/s41594-021-00575-9
- Primary Citation of Related Structures:
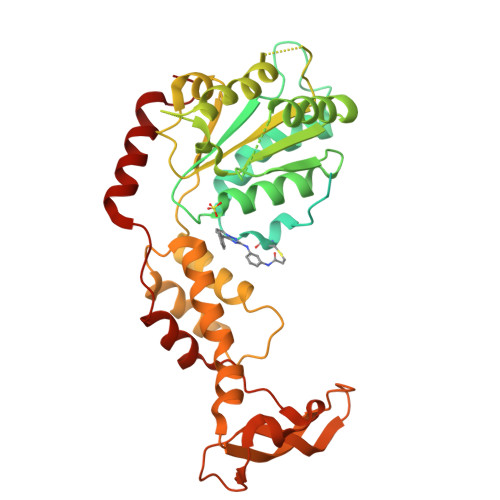
7L9X - PubMed Abstract:
The structural conservation across the AAA (ATPases associated with diverse cellular activities) protein family makes designing selective chemical inhibitors challenging. Here, we identify a triazolopyridine-based fragment that binds the AAA domain of human katanin, a microtubule-severing protein. We have developed a model for compound binding and designed ASPIR-1 (allele-specific, proximity-induced reactivity-based inhibitor-1), a cell-permeable compound that selectively inhibits katanin with an engineered cysteine mutation. Only in cells expressing mutant katanin does ASPIR-1 treatment increase the accumulation of CAMSAP2 at microtubule minus ends, confirming specific on-target cellular activity. Importantly, ASPIR-1 also selectively inhibits engineered cysteine mutants of human VPS4B and FIGL1-AAA proteins, involved in organelle dynamics and genome stability, respectively. Structural studies confirm our model for compound binding at the AAA ATPase site and the proximity-induced reactivity-based inhibition. Together, our findings suggest a chemical genetics approach to decipher AAA protein functions across essential cellular processes and to test hypotheses for developing therapeutics.
Organizational Affiliation:
Laboratory of Chemistry and Cell Biology, The Rockefeller University, New York, NY, USA.