Multiple Substrate Binding Mode-Guided Engineering of a Thermophilic PET Hydrolase.
Pfaff, L., Gao, J., Li, Z., Jackering, A., Weber, G., Mican, J., Chen, Y., Dong, W., Han, X., Feiler, C.G., Ao, Y.F., Badenhorst, C.P.S., Bednar, D., Palm, G.J., Lammers, M., Damborsky, J., Strodel, B., Liu, W., Bornscheuer, U.T., Wei, R.(2022) ACS Catal 12: 9790-9800
- PubMed: 35966606
- DOI: https://doi.org/10.1021/acscatal.2c02275
- Primary Citation of Related Structures:
7CUV, 7E30, 7E31, 7W66, 7W69, 7W6C, 7W6O, 7W6Q - PubMed Abstract:
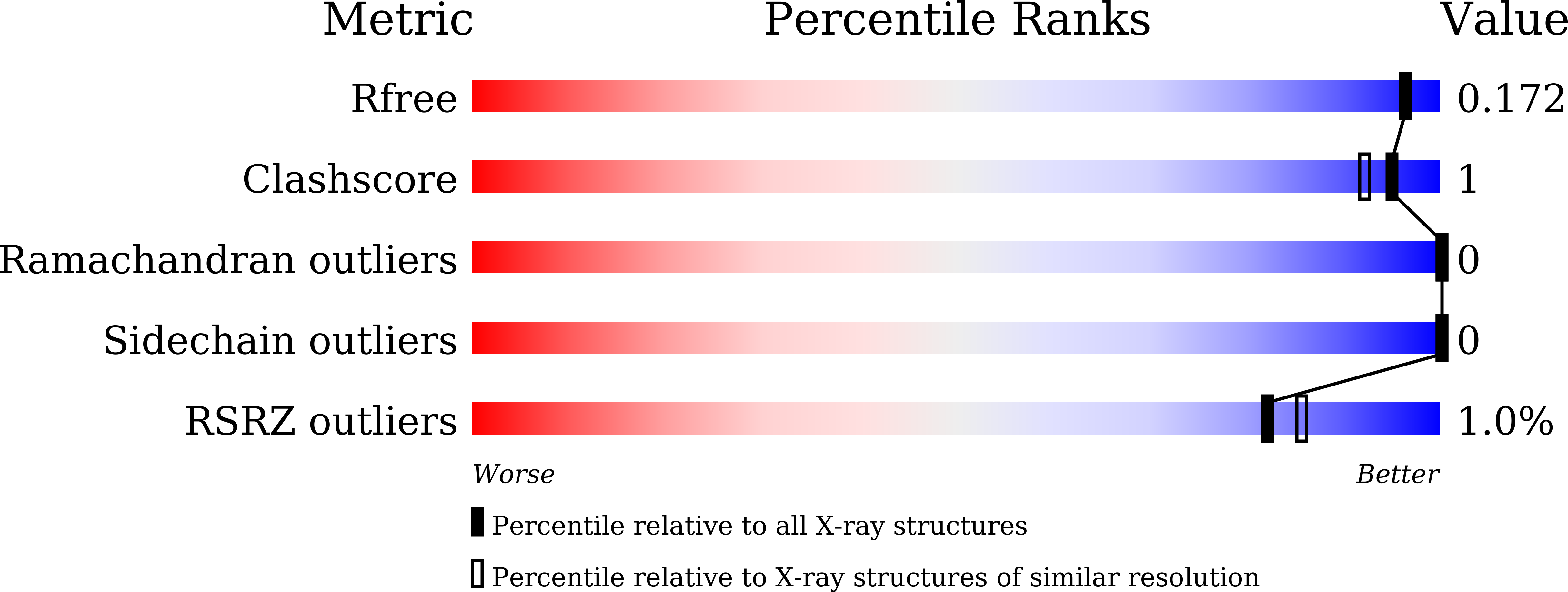
Thermophilic polyester hydrolases (PES-H) have recently enabled biocatalytic recycling of the mass-produced synthetic polyester polyethylene terephthalate (PET), which has found widespread use in the packaging and textile industries. The growing demand for efficient PET hydrolases prompted us to solve high-resolution crystal structures of two metagenome-derived enzymes (PES-H1 and PES-H2) and notably also in complex with various PET substrate analogues. Structural analyses and computational modeling using molecular dynamics simulations provided an understanding of how product inhibition and multiple substrate binding modes influence key mechanistic steps of enzymatic PET hydrolysis. Key residues involved in substrate-binding and those identified previously as mutational hotspots in homologous enzymes were subjected to mutagenesis. At 72 °C, the L92F/Q94Y variant of PES-H1 exhibited 2.3-fold and 3.4-fold improved hydrolytic activity against amorphous PET films and pretreated real-world PET waste, respectively. The R204C/S250C variant of PES-H1 had a 6.4 °C higher melting temperature than the wild-type enzyme but retained similar hydrolytic activity. Under optimal reaction conditions, the L92F/Q94Y variant of PES-H1 hydrolyzed low-crystallinity PET materials 2.2-fold more efficiently than LCC ICCG, which was previously the most active PET hydrolase reported in the literature. This property makes the L92F/Q94Y variant of PES-H1 a good candidate for future applications in industrial plastic recycling processes.
Organizational Affiliation:
Department of Biotechnology & Enzyme Catalysis, Institute of Biochemistry, University of Greifswald, Felix-Hausdorff-Str. 4, 17487 Greifswald, Germany.