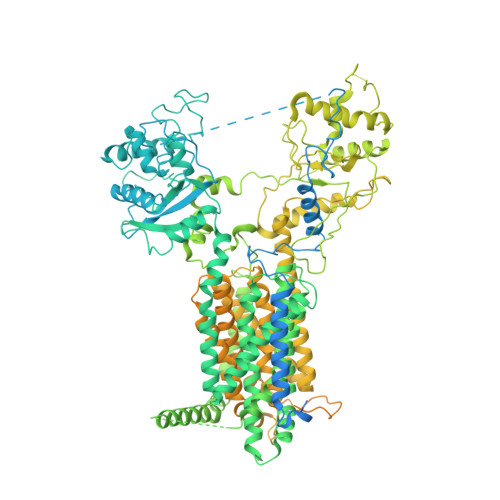
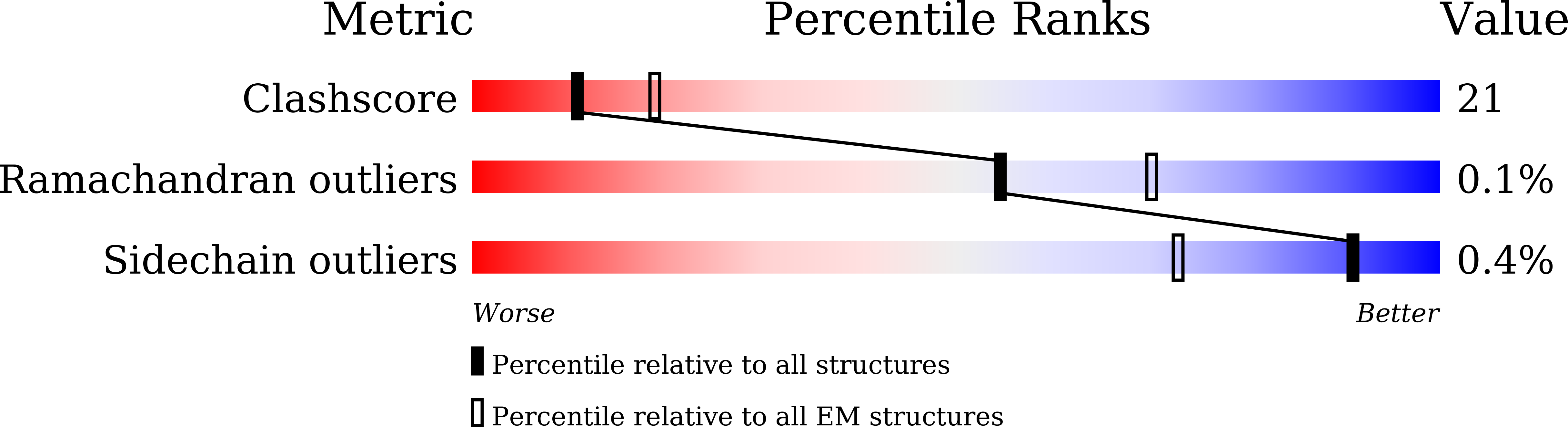Structural insights into proteolytic activation of the human Dispatched1 transporter for Hedgehog morphogen release.
Li, W., Wang, L., Wierbowski, B.M., Lu, M., Dong, F., Liu, W., Li, S., Wang, P., Salic, A., Gong, X.(2021) Nat Commun 12: 6966-6966
- PubMed: 34845226
- DOI: https://doi.org/10.1038/s41467-021-27257-w
- Primary Citation of Related Structures:
7E2G, 7E2H, 7E2I - PubMed Abstract:
The membrane protein Dispatched (Disp), which belongs to the RND family of small molecule transporters, is essential for Hedgehog (Hh) signaling, by catalyzing the extracellular release of palmitate- and cholesterol-modified Hh ligands from producing cells. Disp function requires Furin-mediated proteolytic cleavage of its extracellular domain, but how this activates Disp remains obscure. Here, we employ cryo-electron microscopy to determine atomic structures of human Disp1 (hDisp1), before and after cleavage, and in complex with lipid-modified Sonic hedgehog (Shh) ligand. These structures, together with biochemical data, reveal that proteolytic cleavage opens the extracellular domain of hDisp1, removing steric hindrance to Shh binding. Structure-guided functional experiments demonstrate the role of hDisp1-Shh interactions in ligand release. Our results clarify the mechanisms of hDisp1 activation and Shh morphogen release, and highlight how a unique proteolytic cleavage event enabled acquisition of a protein substrate by a member of a family of small molecule transporters.
Organizational Affiliation:
Department of Biology, School of Life Sciences, Southern University of Science and Technology, 518055, Shenzhen, Guangdong, China.