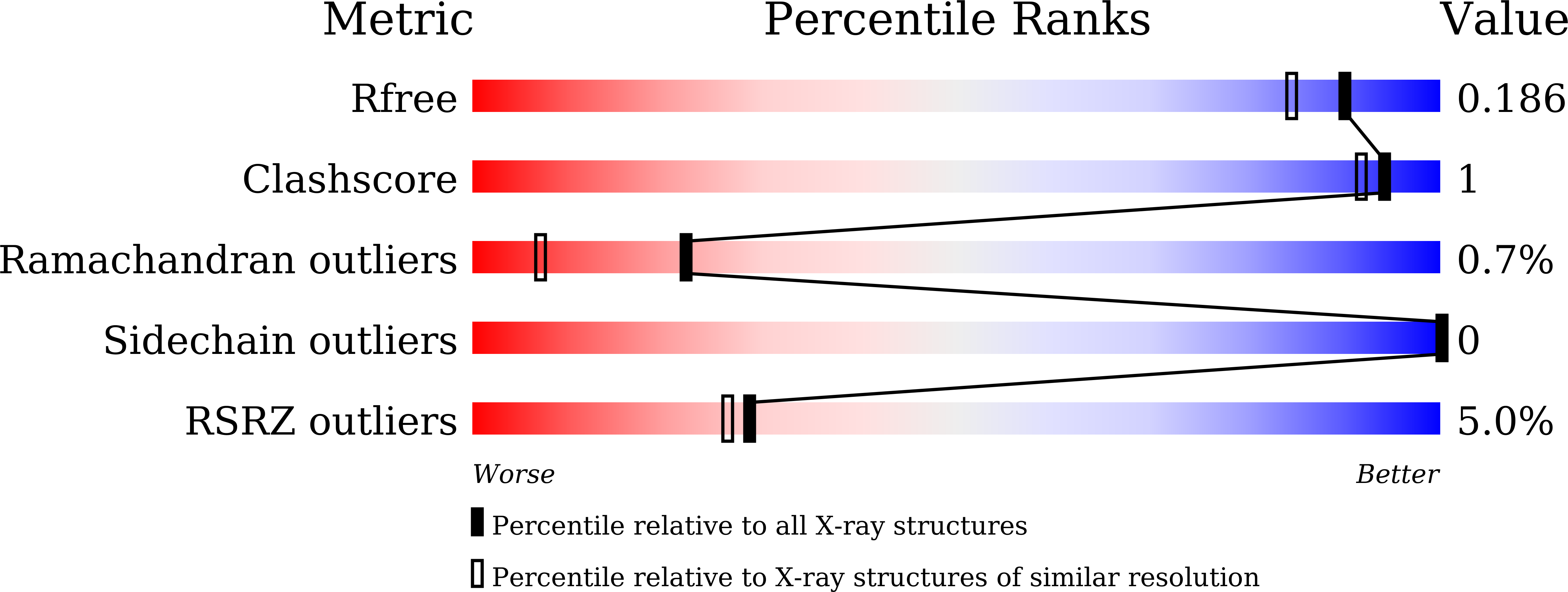
Actin binding to galectin-13/placental protein-13 occurs independently of the galectin canonical ligand-binding site.
Li, X., Yao, Y., Liu, T., Gu, K., Han, Q., Zhang, W., Ayala, G.J., Liu, Y., Na, H., Yu, J., Zhang, F., Mayo, K.H., Su, J.(2021) Glycobiology 31: 1219-1229
- PubMed: 34080003
- DOI: https://doi.org/10.1093/glycob/cwab047
- Primary Citation of Related Structures:
7CYA, 7CYB - PubMed Abstract:
The gene for galectin-13 (Gal-13, placental protein 13) is only present in primates, and its low expression level in maternal serum may promote preeclampsia. In the present study, we used pull-down experiments and biolayer interferometry to assess the interaction between Gal-13 and actin. These studies uncovered that human Gal-13 (hGal-13) and Saimiri boliviensis boliviensis (sGal-13) strongly bind to α- and β-/γ-actin, with Ca2+ and adenosine triphosphate, significantly enhancing the interactions. This in turn suggests that h/sGal-13 may inhibit myosin-induced contraction when vascular smooth muscle cells undergo polarization. Here, we solved the crystal structure of sGal-13 bound to lactose and found that it exists as a monomer in contrast to hGal-13 which is a dimer. The distribution of sGal-13 in HeLa cells is similar to that of hGal-13, indicating that monomeric Gal-13 is the primary form in cells. Even though sGal-13 binds to actin, hGal-13 ligand-binding site mutants do not influence hGal-13/actin binding, whereas the monomeric mutant C136S/C138S binds to actin more strongly than the wild-type hGal-13. Overall, our study demonstrates that monomeric Gal-13 binds to actin, an interaction that is independent of the galectin canonical ligand-binding site.
Organizational Affiliation:
Engineering Research Center of Glycoconjugates Ministry of Education, Jilin Provincial Key Laboratory of Chemistry and Biology of Changbai Mountain Natural Drugs, School of Life Sciences, Northeast Normal University, Changchun 130024, China.