Mechanistic Insights into the Interactions of Ras Subfamily GTPases with the SPN Domain of Autism-associated SHANK3.
Xu, X.L., Liu, J.P., Wang, Y.L., Wang, Y., Gong, X., Pan, L.F.(2020) Chin J Chem 38: 1635-1641
Experimental Data Snapshot
Starting Models: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Ras-related protein Rap-1b | 167 | Homo sapiens | Mutation(s): 2 Gene Names: RAP1B, OK/SW-cl.11 EC: 3.6.5.2 | 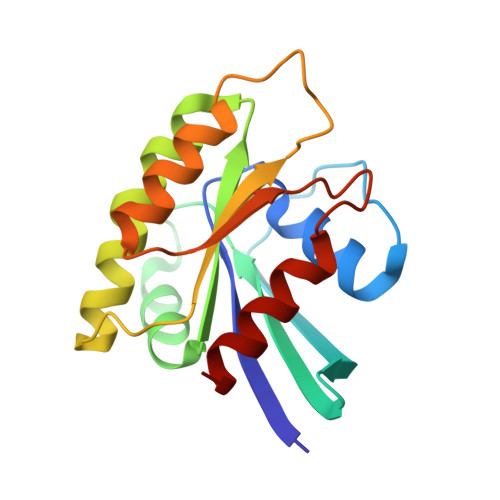 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P61224 (Homo sapiens) Explore P61224 Go to UniProtKB: P61224 | |||||
PHAROS: P61224 GTEx: ENSG00000127314 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P61224 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| SH3 and multiple ankyrin repeat domains protein 3 | 99 | Homo sapiens | Mutation(s): 0 Gene Names: SHANK3, KIAA1650, PROSAP2, PSAP2 | 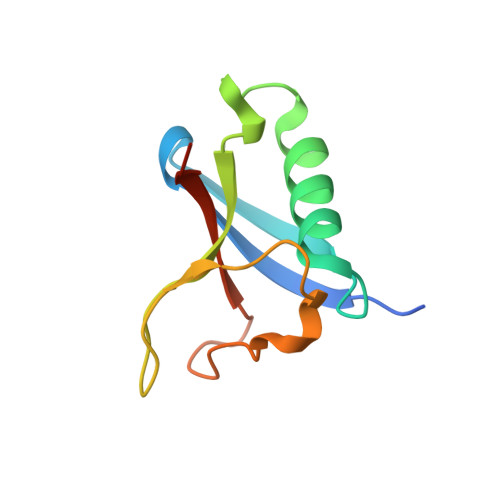 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q9BYB0 (Homo sapiens) Explore Q9BYB0 Go to UniProtKB: Q9BYB0 | |||||
PHAROS: Q9BYB0 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9BYB0 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| GTP (Subject of Investigation/LOI) Query on GTP | E [auth A], H [auth B] | GUANOSINE-5'-TRIPHOSPHATE C10 H16 N5 O14 P3 XKMLYUALXHKNFT-UUOKFMHZSA-N |  | ||
| CA Query on CA | F [auth A] | CALCIUM ION Ca BHPQYMZQTOCNFJ-UHFFFAOYSA-N |  | ||
| MG (Subject of Investigation/LOI) Query on MG | G [auth A], I [auth B] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 58.215 | α = 90 |
| b = 191.926 | β = 90 |
| c = 48.876 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| PHENIX | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Natural Science Foundation of China (NSFC) | China | 21822705 |