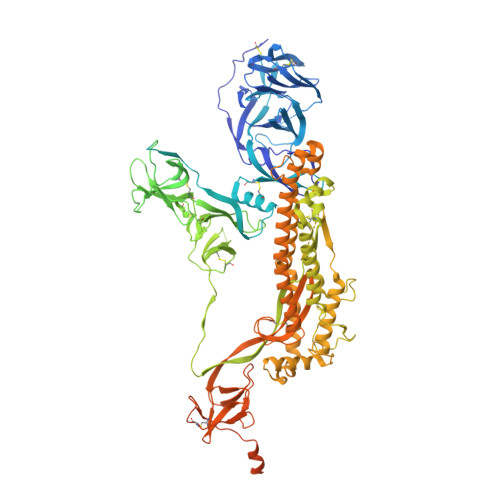
Structural analysis of receptor binding domain mutations in SARS-CoV-2 variants of concern that modulate ACE2 and antibody binding.
Mannar, D., Saville, J.W., Zhu, X., Srivastava, S.S., Berezuk, A.M., Zhou, S., Tuttle, K.S., Kim, A., Li, W., Dimitrov, D.S., Subramaniam, S.(2021) Cell Rep 37: 110156-110156
- PubMed: 34914928
- DOI: https://doi.org/10.1016/j.celrep.2021.110156
- Primary Citation of Related Structures:
7SXR, 7SXS, 7SXT, 7SXU, 7SXV, 7SXW, 7SXX, 7SXY, 7SXZ, 7SY0, 7SY1, 7SY2, 7SY3, 7SY4, 7SY5, 7SY6, 7SY7, 7SY8 - PubMed Abstract:
The recently emerged severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) Beta (B.1.351) and Gamma (P.1) variants of concern (VoCs) include a key mutation (N501Y) found in the Alpha (B.1.1.7) variant that enhances affinity of the spike protein for its receptor, angiotensin-converting enzyme 2 (ACE2). Additional mutations are found in these variants at residues 417 and 484 that appear to promote antibody evasion. In contrast, the Epsilon variants (B.1.427/429) lack the N501Y mutation yet exhibit antibody evasion. We have engineered spike proteins to express these receptor binding domain (RBD) VoC mutations either in isolation or in different combinations and analyze the effects using biochemical assays and cryoelectron microscopy (cryo-EM) structural analyses. Overall, our findings suggest that the emergence of new SARS-CoV-2 variant spikes can be rationalized as the result of mutations that confer increased ACE2 affinity, increased antibody evasion, or both, providing a framework to dissect the molecular factors that drive VoC evolution.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, University of British Columbia, Vancouver, BC V6T 1Z3, Canada.