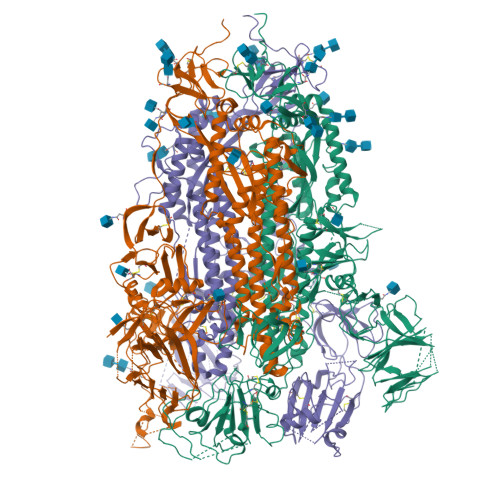
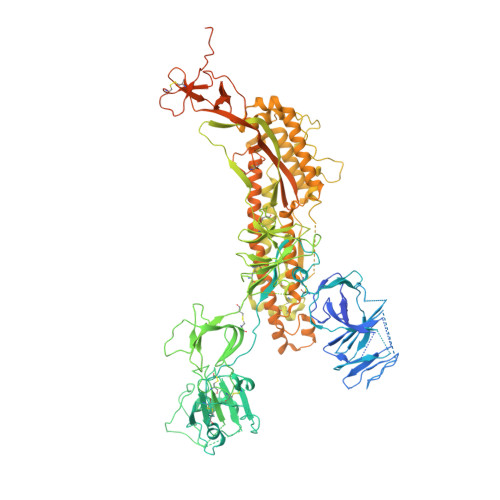
SARS-CoV-2 S2P spike ages through distinct states with altered immunogenicity.
Olia, A.S., Tsybovsky, Y., Chen, S.J., Liu, C., Nazzari, A.F., Ou, L., Wang, L., Kong, W.P., Leung, K., Liu, T., Stephens, T., Teng, I.T., Wang, S., Yang, E.S., Zhang, B., Zhang, Y., Zhou, T., Mascola, J.R., Kwong, P.D.(2021) J Biological Chem 297: 101127-101127
- PubMed: 34461095
- DOI: https://doi.org/10.1016/j.jbc.2021.101127
- Primary Citation of Related Structures:
7MTC, 7MTD, 7MTE - PubMed Abstract:
The SARS-CoV-2 spike is the primary target of virus-neutralizing antibodies and critical to the development of effective vaccines against COVID-19. Here, we demonstrate that the prefusion-stabilized two-proline "S2P" spike-widely employed for laboratory work and clinical studies-unfolds when stored at 4 °C, physiological pH, as observed by electron microscopy (EM) and differential scanning calorimetry, but that its trimeric, native-like conformation can be reacquired by low pH treatment. When stored for approximately 1 week, this unfolding does not significantly alter antigenic characteristics; however, longer storage diminishes antibody binding, and month-old spike elicits virtually no neutralization in mice despite inducing high ELISA-binding titers. Cryo-EM structures reveal the folded fraction of spike to decrease with aging; however, its structure remains largely similar, although with varying mobility of the receptor-binding domain. Thus, the SARS-CoV-2 spike is susceptible to unfolding, which affects immunogenicity, highlighting the need to monitor its integrity.
Organizational Affiliation:
Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.