Comprehensive Strategies to Bicyclic Prolines: Applications in the Synthesis of Potent Arginase Inhibitors.
Li, D., Zhang, H., Lyons, T.W., Lu, M., Achab, A., Pu, Q., Childers, M., Mitcheltree, M.J., Wang, J., Martinot, T.A., McMinn, S.E., Sloman, D.L., Palani, A., Beard, A., Nogle, L., Gathiaka, S., Sauri, J., Kim, H.Y., Adpressa, D., Spacciapoli, P., Miller, J.R., Palte, R.L., Lesburg, C.A., Cumming, J., Fischer, C.(2021) ACS Med Chem Lett 12: 1678-1688
- PubMed: 34795856
- DOI: https://doi.org/10.1021/acsmedchemlett.1c00258
- Primary Citation of Related Structures:
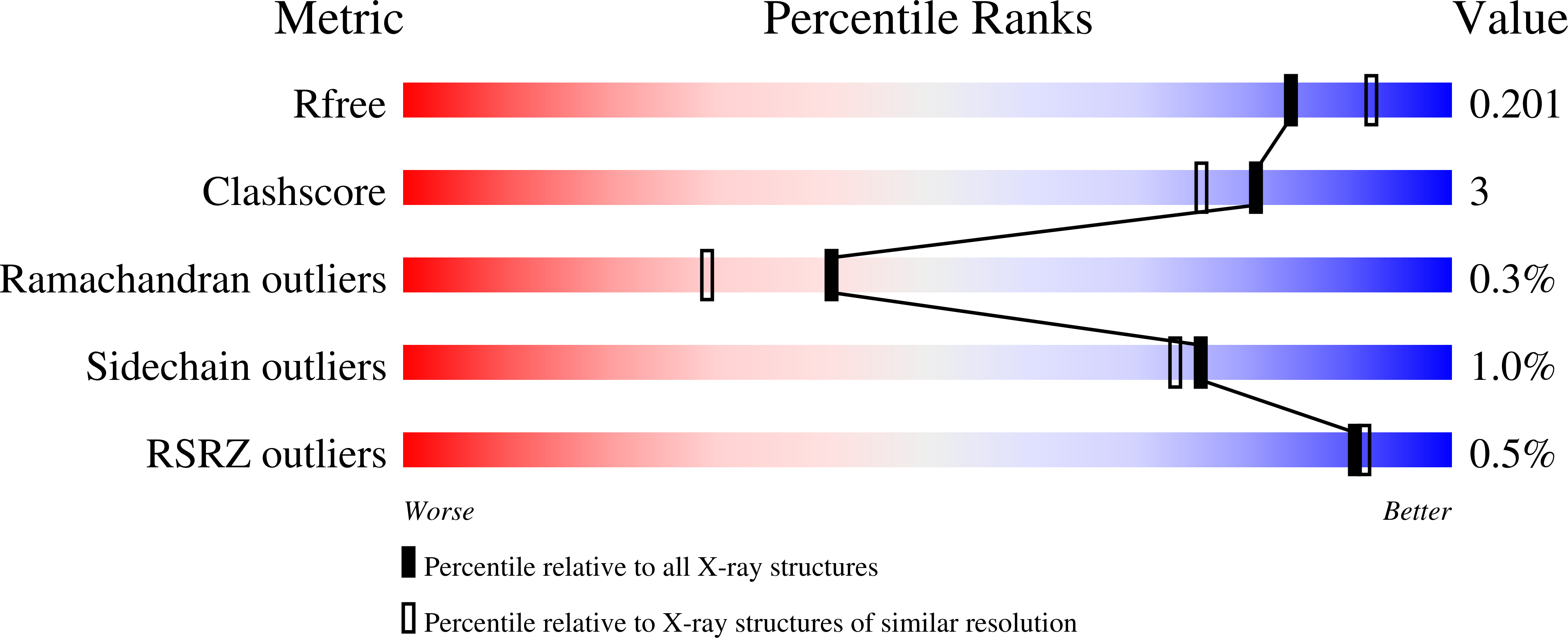
7K4G, 7K4H, 7K4I, 7K4J, 7K4K - PubMed Abstract:
Comprehensive synthetic strategies afforded a diverse set of structurally unique bicyclic proline-containing arginase inhibitors with a high degree of three-dimensionality. The analogs that favored the Cγ-exo conformation of the proline improved the arginase potency over the initial lead. The novel synthetic strategies reported here not only enable access to previously unknown stereochemically complex proline derivatives but also provide a foundation for the future synthesis of bicyclic proline analogs, which incorporate inherent three-dimensional character into building blocks, medicine, and catalysts and could have a profound impact on the conformation of proline-containing peptides and macrocycles.
Organizational Affiliation:
Department of Discovery Chemistry, Department of Discovery Process Chemistry, Department of In Vitro Pharmacology, Department of Computational and Structural Chemistry, and Department of Analytical Research and Development, Merck & Co., Inc., 33 Avenue Louis Pasteur, Boston, Massachusetts 02115, United States.