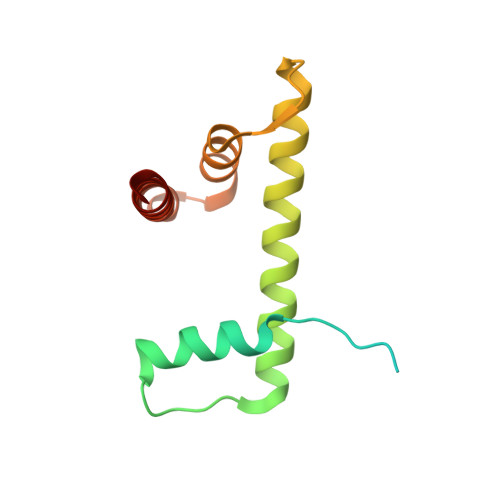
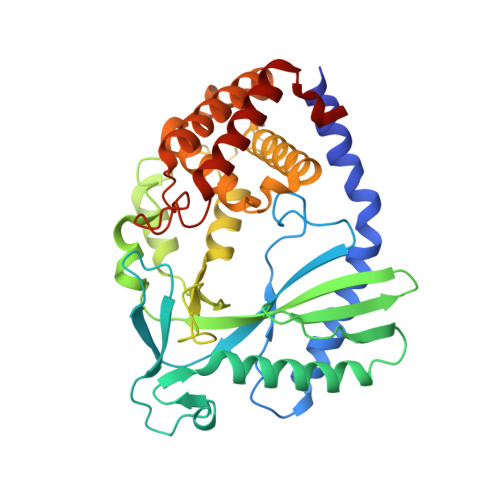
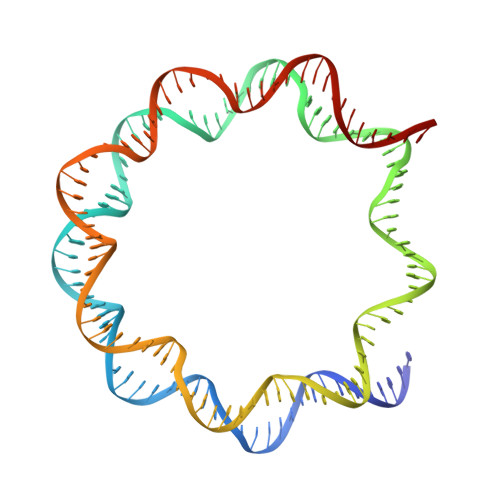
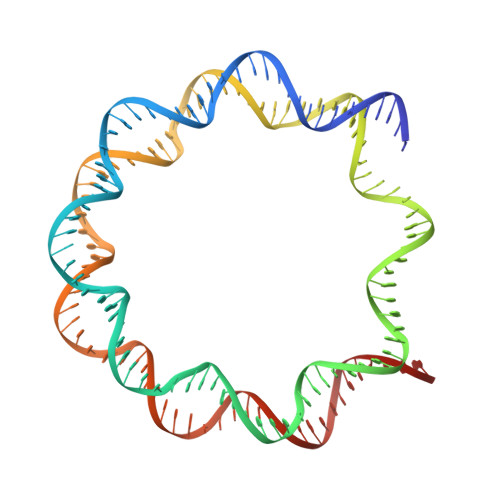
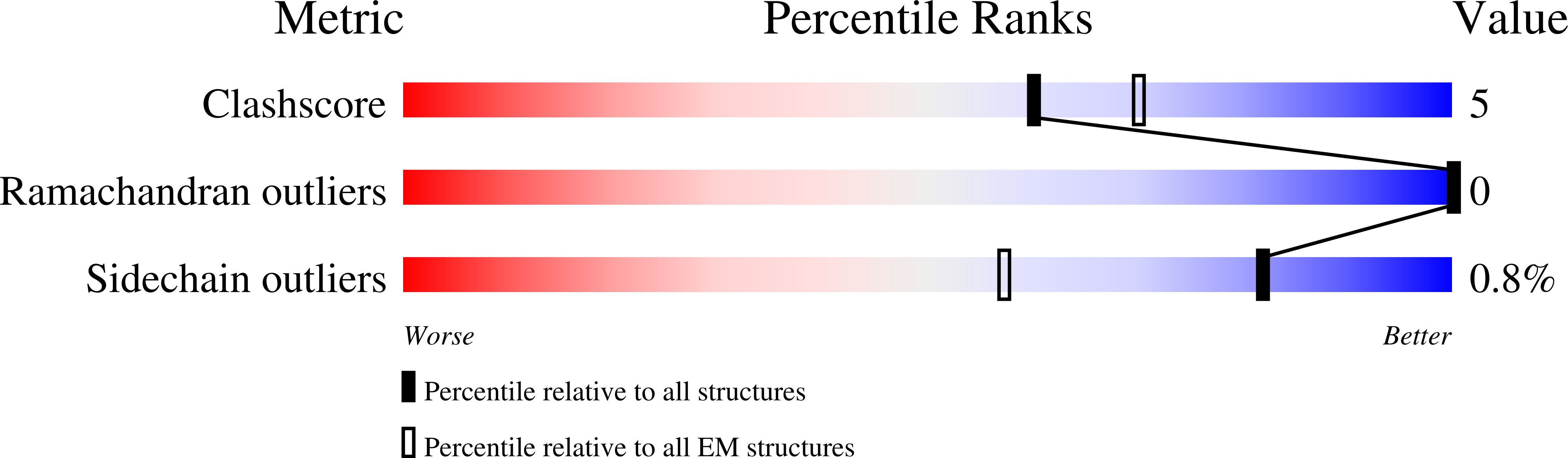Structural basis of nucleosome-dependent cGAS inhibition.
Boyer, J.A., Spangler, C.J., Strauss, J.D., Cesmat, A.P., Liu, P., McGinty, R.K., Zhang, Q.(2020) Science 370: 450-454
- PubMed: 32913000
- DOI: https://doi.org/10.1126/science.abd0609
- Primary Citation of Related Structures:
7JO9, 7JOA - PubMed Abstract:
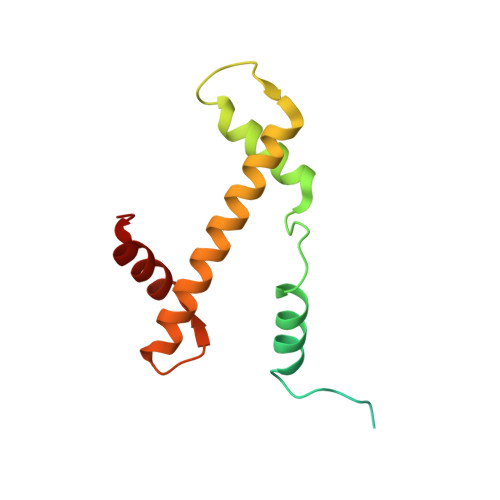
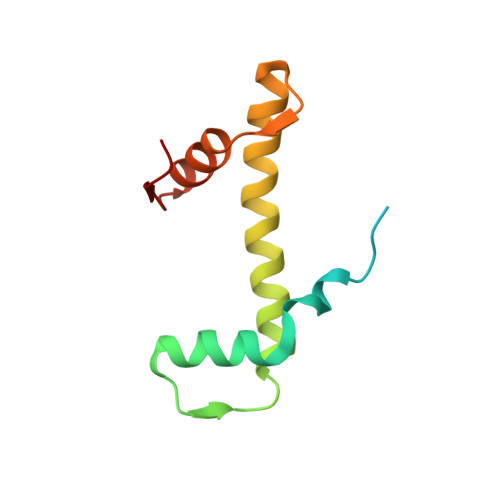
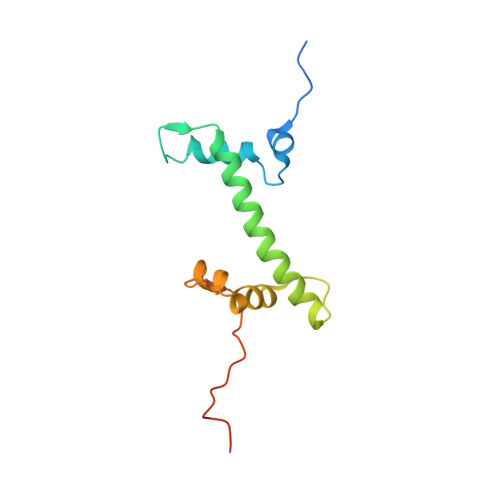
Cyclic guanosine monophosphate (GMP)-adenosine monophosphate (AMP) synthase (cGAS) recognizes cytosolic foreign or damaged DNA to activate the innate immune response to infection, inflammatory diseases, and cancer. By contrast, cGAS reactivity against self-DNA in the nucleus is suppressed by chromatin tethering. We report a 3.3-angstrom-resolution cryo-electron microscopy structure of cGAS in complex with the nucleosome core particle. The structure reveals that cGAS uses two conserved arginines to anchor to the nucleosome acidic patch. The nucleosome-binding interface exclusively occupies the strong double-stranded DNA (dsDNA)-binding surface on cGAS and sterically prevents cGAS from oligomerizing into the functionally active 2:2 cGAS-dsDNA state. These findings provide a structural basis for how cGAS maintains an inhibited state in the nucleus and further exemplify the role of the nucleosome in regulating diverse nuclear protein functions.
Organizational Affiliation:
Department of Biochemistry and Biophysics, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.