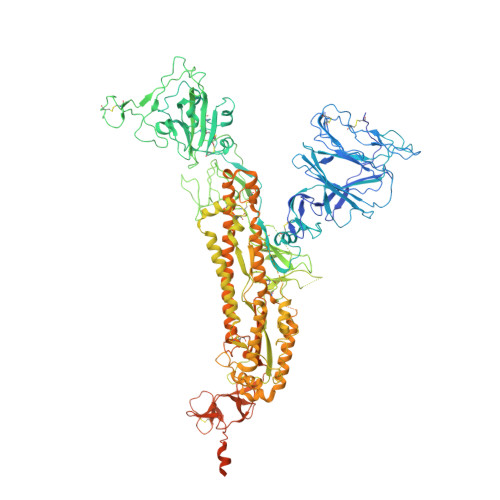
The effect of the D614G substitution on the structure of the spike glycoprotein of SARS-CoV-2.
Benton, D.J., Wrobel, A.G., Roustan, C., Borg, A., Xu, P., Martin, S.R., Rosenthal, P.B., Skehel, J.J., Gamblin, S.J.(2021) Proc Natl Acad Sci U S A 118
- PubMed: 33579792
- DOI: https://doi.org/10.1073/pnas.2022586118
- Primary Citation of Related Structures:
7BNM, 7BNN, 7BNO - PubMed Abstract:
The majority of currently circulating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viruses have mutant spike glycoproteins that contain the D614G substitution. Several studies have suggested that spikes with this substitution are associated with higher virus infectivity. We use cryo-electron microscopy to compare G614 and D614 spikes and show that the G614 mutant spike adopts a range of more open conformations that may facilitate binding to the SARS-CoV-2 receptor, ACE2, and the subsequent structural rearrangements required for viral membrane fusion.
Organizational Affiliation:
Structural Biology of Disease Processes Laboratory, Francis Crick Institute, London NW1 1AT, United Kingdom; Donald.Benton@crick.ac.uk John.Skehel@crick.ac.uk Antoni.Wrobel@crick.ac.uk Steve.Gamblin@crick.ac.uk.