Xanthopsin-Like Systems via Site-Specific Click-Functionalization of a Retinoic Acid Binding Protein.
Tassone, G., Paolino, M., Pozzi, C., Reale, A., Salvini, L., Giorgi, G., Orlandini, M., Galvagni, F., Mangani, S., Yang, X., Carlotti, B., Ortica, F., Latterini, L., Olivucci, M., Cappelli, A.(2022) Chembiochem 23: e202100449-e202100449
- PubMed: 34647400
- DOI: https://doi.org/10.1002/cbic.202100449
- Primary Citation of Related Structures:
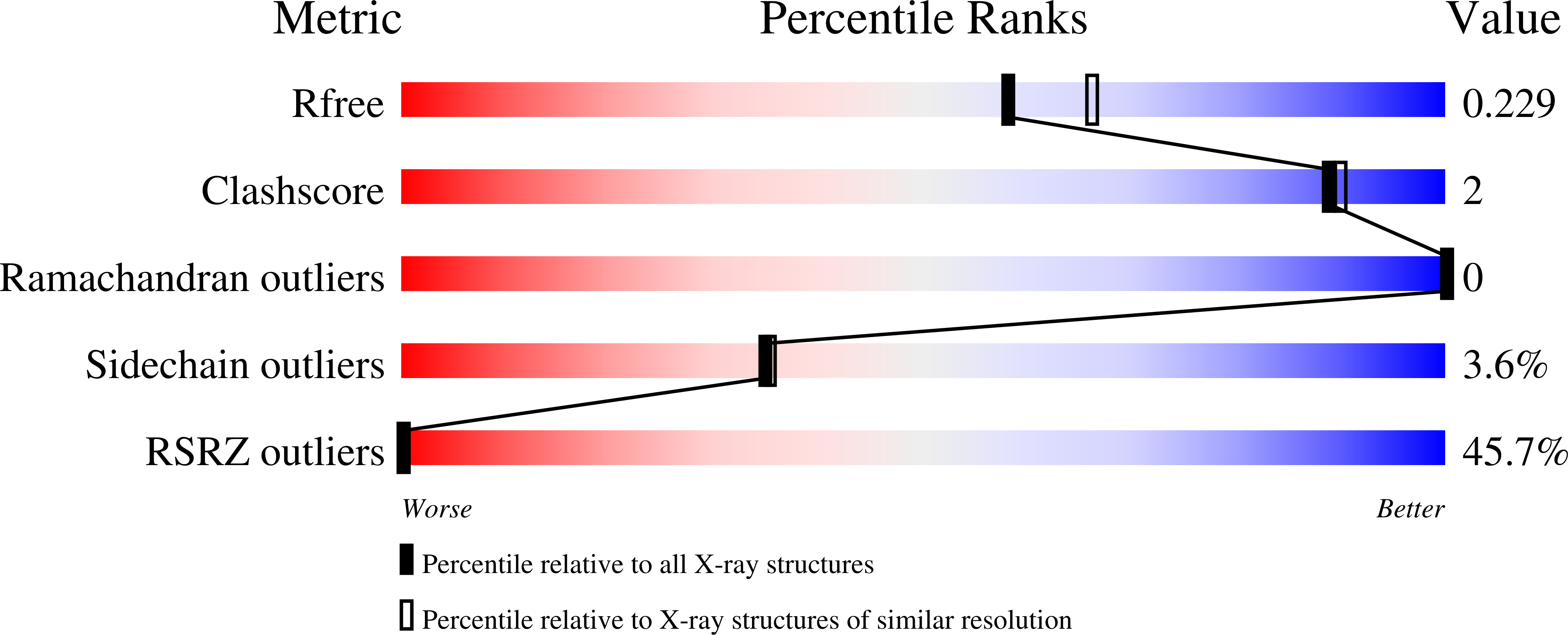
6Z2U, 6Z2Z, 6ZSW, 6ZSX - PubMed Abstract:
The use of light-responsive proteins to control both living or synthetic cells, is at the core of the expanding fields of optogenetics and synthetic biology. It is thus apparent that a richer reaction toolbox for the preparation of such systems is of fundamental importance. Here, we provide a proof-of-principle demonstration that Morita-Baylis-Hillman adducts can be employed to perform a facile site-specific, irreversible and diastereoselective click-functionalization of a lysine residue buried into a lipophilic binding pocket and yielding an unnatural chromophore with an extended π-system. In doing so we effectively open the path to the in vitro preparation of a library of synthetic proteins structurally reminiscent of xanthopsin eubacterial photoreceptors. We argue that such a library, made of variable unnatural chromophores inserted in an easy-to-mutate and crystallize retinoic acid transporter, significantly expand the scope of the recently introduced rhodopsin mimics as both optogenetic and "lab-on-a-molecule" tools.
Organizational Affiliation:
Dipartimento di Biotecnologie, Chimica e Farmacia, Dipartimento di Eccellenza 2018-2022), Università degli Studi di Siena, Via A. Moro 2, 53100, Siena, Italy.