Direct Introduction of an Alkylsulfonamido Group on C-sites of Isomeric Dicarba-closo-dodecaboranes: The Influence of Stereochemistry on Inhibitory Activity against the Cancer-Associated Carbonic Anhydrase IX Isoenzyme.
Nekvinda, J., Kugler, M., Holub, J., El Anwar, S., Brynda, J., Pospisilova, K., Ruzickova, Z., Rezacova, P., Gruner, B.(2020) Chemistry 26: 16541-16553
- PubMed: 32757220
- DOI: https://doi.org/10.1002/chem.202002809
- Primary Citation of Related Structures:
6YO2, 6YO4, 6YO7, 6YOI, 6YOK, 6YOL - PubMed Abstract:
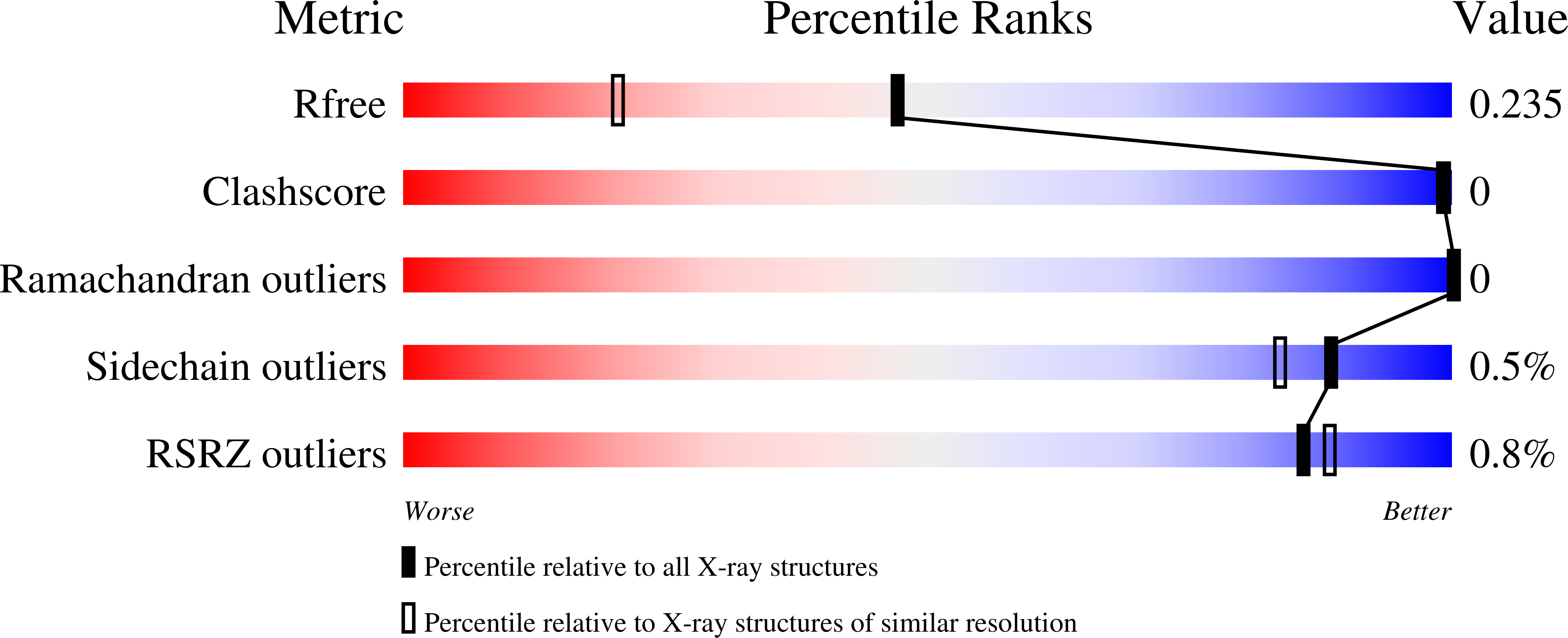
Carbonic anhydrase IX (CA IX), a tumor-associated metalloenzyme, represents a validated target for cancer therapy and diagnostics. Herein, we report the inhibition properties of isomeric families of sulfonamidopropyl-dicarba-closo-dodecaboranes group(s) prepared using a new direct five-step synthesis from the corresponding parent cages. The protocol offers a reliable solution for synthesis of singly and doubly substituted dicarba-closo-dodecaboranes with a different geometric position of carbon atoms. The closo-compounds from the ortho- and meta-series were then degraded to corresponding 11-vertex dicarba-nido-undecaborate(1-) anions. All compounds show in vitro enzymatic activity against CA IX in the low nanomolar or subnanomolar range. This is accompanied by clear isomer dependence of the inhibition constant (K i ) and selectivity towards CA IX. Decreasing trends in K i and selectivity index (S I ) values are observed with increasing separation of the cage carbon atoms. Interactions of compounds with the active sites of CA IX were explored with X-ray crystallography, and eight high-resolution crystal structures uncovered the structural basis of inhibition potency and selectivity.
Organizational Affiliation:
Department of Synthesis, Institute of Inorganic Chemistry of, the Czech Academy of Sciences, 25068, Řež, Czech Republic.