Crystal structure of CD32b (Fc Gamma Receptor IIb) in complex with Human IgG1 Fab fragment (5C05)
Fisher, H., Tews, I., Orr, C.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| 5C05 F(ab) heavy chain | A [auth HHH], E [auth III] | 218 | Homo sapiens | Mutation(s): 0 | 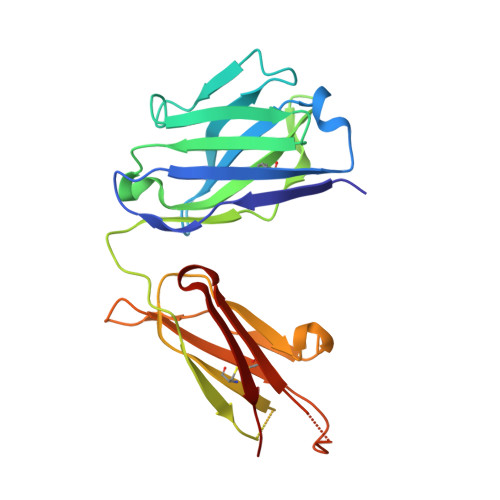 |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| 5C05 F(ab) light chain | B [auth MMM], D [auth LLL] | 218 | Homo sapiens | Mutation(s): 0 | 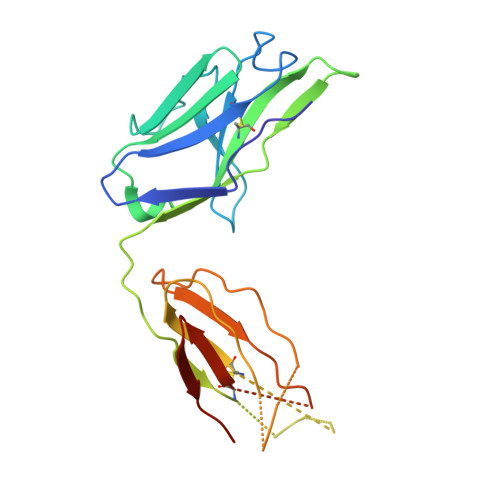 |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Low affinity immunoglobulin gamma Fc region receptor II-c | C [auth AAA], F [auth BBB] | 181 | Homo sapiens | Mutation(s): 0 Gene Names: FCGR2C, CD32, FCG2, IGFR2 | 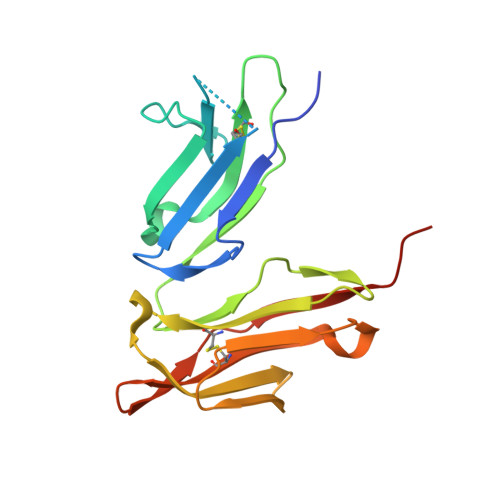 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P31995 (Homo sapiens) Explore P31995 Go to UniProtKB: P31995 | |||||
PHAROS: P31995 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P31995 | ||||
Glycosylation | |||||
| Glycosylation Sites: 1 | Go to GlyGen: P31995-1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| NAG Query on NAG | I [auth AAA] | 2-acetamido-2-deoxy-beta-D-glucopyranose C8 H15 N O6 OVRNDRQMDRJTHS-FMDGEEDCSA-N |  | ||
| PG4 Query on PG4 | H [auth HHH], K [auth III] | TETRAETHYLENE GLYCOL C8 H18 O5 UWHCKJMYHZGTIT-UHFFFAOYSA-N |  | ||
| GOL Query on GOL | J [auth LLL] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 95.728 | α = 90 |
| b = 95.728 | β = 90 |
| c = 186.038 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| DIALS | data reduction |
| Aimless | data scaling |
| MOLREP | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Cancer Research UK | United Kingdom | S_3591 |