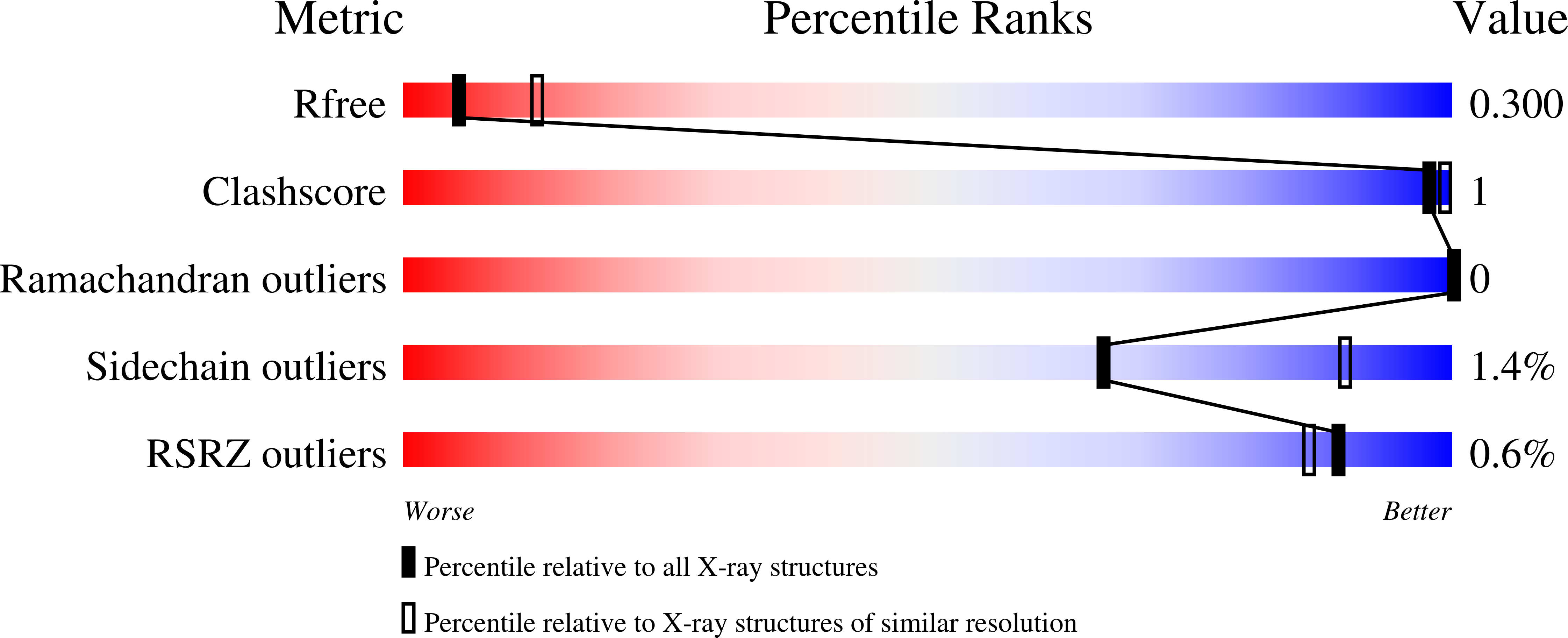
Selective inhibition of cullin 3 neddylation through covalent targeting DCN1 protects mice from acetaminophen-induced liver toxicity.
Zhou, H., Lu, J., Chinnaswamy, K., Stuckey, J.A., Liu, L., McEachern, D., Yang, C.Y., Bernard, D., Shen, H., Rui, L., Sun, Y., Wang, S.(2021) Nat Commun 12: 2621-2621
- PubMed: 33976147
- DOI: https://doi.org/10.1038/s41467-021-22924-4
- Primary Citation of Related Structures:
6XOL, 6XOM, 6XON, 6XOO, 6XOP, 6XOQ - PubMed Abstract:
Cullin-RING E3 ligases (CRLs) regulate the turnover of approximately 20% of mammalian cellular proteins. Neddylation of individual cullin proteins is essential for the activation of each CRL. We report herein the discovery of DI-1548 and DI-1859 as two potent, selective and covalent DCN1 inhibitors. These inhibitors selectively inhibit neddylation of cullin 3 in cells at low nanomolar concentrations and are 2-3 orders of magnitude more potent than our previously reported reversible DCN1 inhibitor. Mass spectrometric analysis and co-crystal structures reveal that these compounds employ a unique mechanism of covalent bond formation with DCN1. DI-1859 induces a robust increase of NRF2 protein, a CRL3 substrate, in mouse liver and effectively protects mice from acetaminophen-induced liver damage. Taken together, this study demonstrates the therapeutic potential of selective inhibition of cullin neddylation.
Organizational Affiliation:
Department of Internal Medicine, University of Michigan, Ann Arbor, MI, USA.