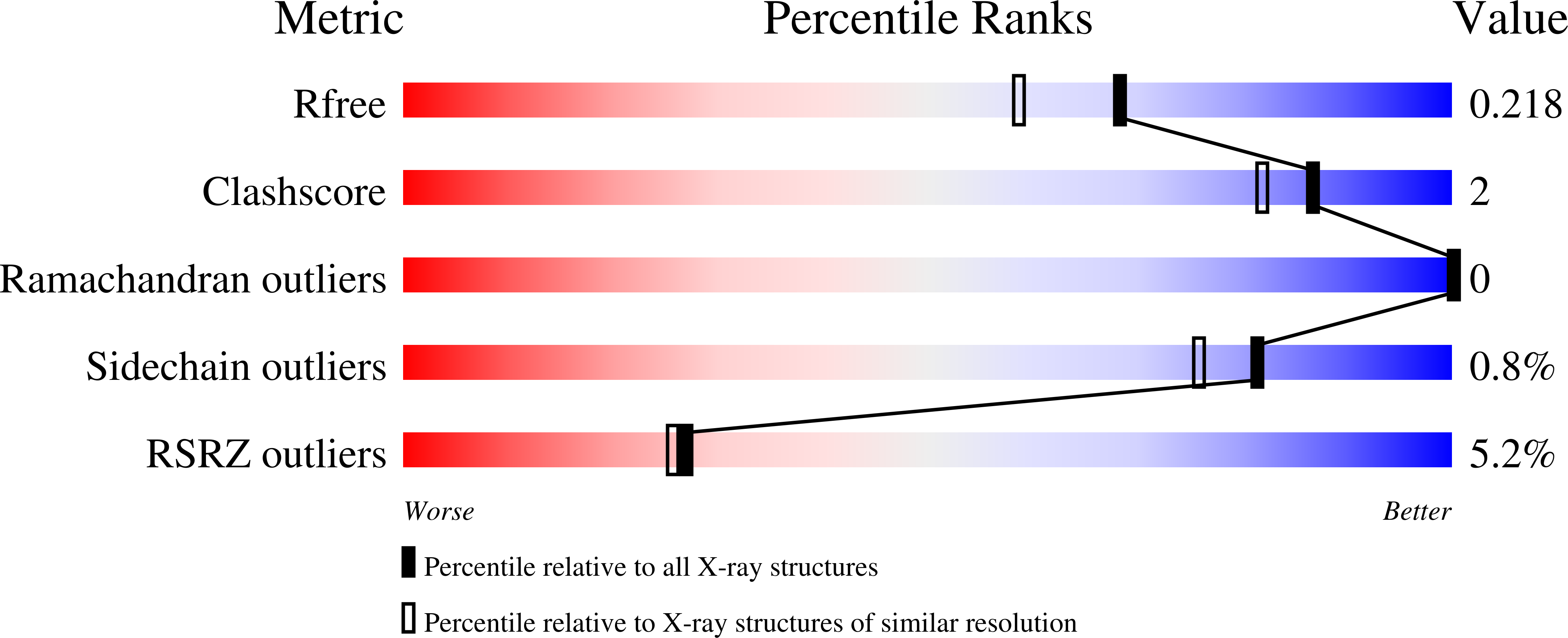
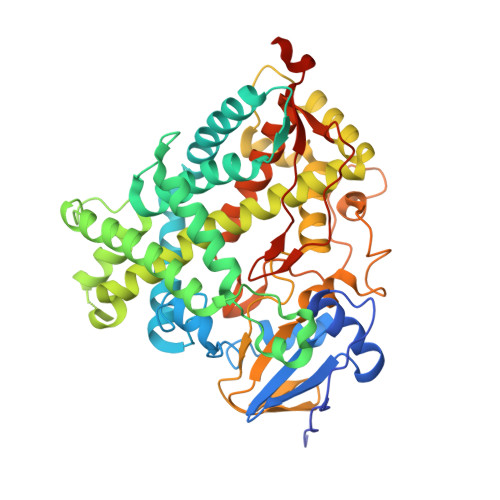
Human cytochrome P450 17A1 structures with metabolites of prostate cancer drug abiraterone reveal substrate-binding plasticity and a second binding site.
Petrunak, E.M., Bart, A.G., Peng, H.M., Auchus, R.J., Scott, E.E.(2023) J Biol Chem 299: 102999-102999
- PubMed: 36773804
- DOI: https://doi.org/10.1016/j.jbc.2023.102999
- Primary Citation of Related Structures:
5UYS, 6WR0, 6WR1, 6WW0 - PubMed Abstract:
Abiraterone acetate is a first-line therapy for castration-resistant prostate cancer. This prodrug is deacetylated in vivo to abiraterone, which is a potent and specific inhibitor of cytochrome P450 17A1 (CYP17A1). CYP17A1 performs two sequential steps that are required for the biosynthesis of androgens that drive prostate cancer proliferation, analogous to estrogens in breast cancer. Abiraterone can be further metabolized in vivo on the steroid A ring to multiple metabolites that also inhibit CYP17A1. Despite its design as an active-site-directed substrate analog, abiraterone and its metabolites demonstrate mixed competitive/noncompetitive inhibition. To understand their binding, we solved the X-ray structures of CYP17A1 with three primary abiraterone metabolites. Despite different conformations of the steroid A ring and substituents, all three bound in the CYP17A1 active site with the steroid core packed against the I helix and the A ring C3 keto or hydroxyl oxygen forming a hydrogen bond with N202 similar to abiraterone itself. The structure of CYP17A1 with 3-keto, 5α-abiraterone was solved to 2.0 Å, the highest resolution to date for a CYP17A1 complex. This structure had additional electron density near the F/G loop, which is likely a second molecule of the inhibitor and which may explain the noncompetitive inhibition. Mutation of the adjacent Asn52 to Tyr positions its side chain in this space, maintains enzyme activity, and prevents binding of the peripheral ligand. Collectively, our findings provide further insight into abiraterone metabolite binding and CYP17A1 function.
Organizational Affiliation:
Department of Medicinal Chemistry, University of Kansas, Lawrence, Kansas, USA.